- Title
-
Anteroposterior patterning of the zebrafish ear through Fgf- and Hh-dependent regulation of hmx3a expression
- Authors
- Hartwell, R.D., England, S.J., Monk, N.A.M., van Hateren, N.J., Baxendale, S., Marzo, M., Lewis, K.E., Whitfield, T.T.
- Source
- Full text @ PLoS Genet.
|
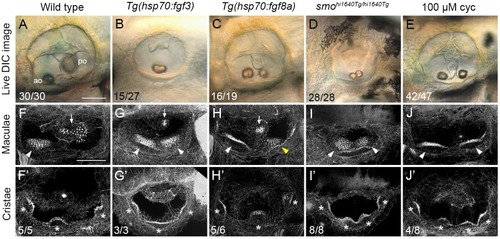
(A–E) Differential interference contrast (DIC) images of ears in live embryos at 3 dpf (72 hpf). (F–J’) Confocal images of FITC-phalloidin stains, revealing stereociliary bundles on sensory hair cells in the maculae (F–J) or cristae (F’–J’). Anterior maculae and duplicate anterior maculae are marked with arrowheads; posterior maculae and remnants of posterior maculae are marked with arrows. Cristae and duplicate cristae are marked with asterisks. Yellow arrowhead in H indicates macula that is ventromedial in position, and close to remnants of the posterior macula (arrow). Note the enlarged lateral crista in G’. (The bright spot in the centre of F’ is a lateral line neuromast.) Representative phenotypes are shown; numbers of embryos displaying these phenotypes are indicated on the panels. All PHENOTYPE:
|
|
In situ hybridisation of otic expression patterns in EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of mRNA for anterior otic markers in embryos treated with 100 μM cyclopamine (cyc) from the 10-somite stage (14 hpf) until 22.5 hpf. Controls in the left-hand panels of each pair of images were treated with vehicle (ethanol) only. (A–F’) At 22.5 hpf (8.5 hours post initiation of treatment, hpt), expression of EXPRESSION / LABELING:
PHENOTYPE:
|
|
(A–F’) In situ hybridisation for otic expression of EXPRESSION / LABELING:
PHENOTYPE:
|
|
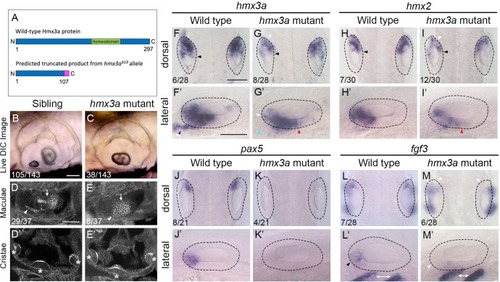
(A) Schematic diagram showing the predicted truncated product for the EXPRESSION / LABELING:
PHENOTYPE:
|
|
(A–D) Control experiments to check for successful expression of the EXPRESSION / LABELING:
PHENOTYPE:
|