- Title
-
penner/lgl2 is required for the integrity of the photoreceptor layer in the zebrafish retina
- Authors
- Kujawski, S., Sonawane, M., Knust, E.
- Source
- Full text @ Biol. Open
|
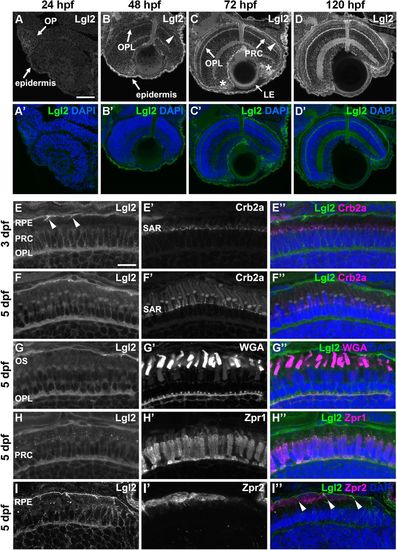
Lgl2 expression is upregulated in the retina by 72 dpf and localizes basolaterally in photoreceptor and RPE cells.Immunostaining of transverse retinal sections. (A–D′) Lgl2 expression during retinal development: A,A′, 24 hpf; B,B′, 48 hpf; C,C′, 72 hpf; D,D′, 120 hpf. Lgl2 expression is upregulated by 72 hpf in the RPE (C, arrowhead) and the OPL. Asterisks in C denote expression in the ciliary marginal zone (CMZ). OP, olfactory placode; OPL, outer plexiform layer; PRC, photoreceptor cell layer; LE, lens epithelium. Scale bar: 50 µm. (E–E″) At 3 dpf, Lgl2 (E) localizes basolaterally in the PRC and in the RPE. Lgl2 staining does not overlap with that of Crb2a (E′), which localizes to the subapical region (SAR). Arrowheads in E denote lateral Lgl2 localization in RPE cells. E″ shows merged image. (F–I″) Lgl2 localization at 5 dpf. (F–F″) Co-staining of Lgl2 with Crb2a shows that Lgl2 staining remains basolateral as PRCs mature. (G–H″) Co-staining of Lgl2 (G,H) with WGA (G′) or Zpr1 (H′) illustrates Lgl2 localization in the OPL. (I–I″) Co-staining of Lgl2 (I) with Zpr2 (I′) reveals basolateral expression in the RPE (arrowheads in I″ denote lateral localization). OS, outer segments. Scale bar: 10 µm. EXPRESSION / LABELING:
|
|
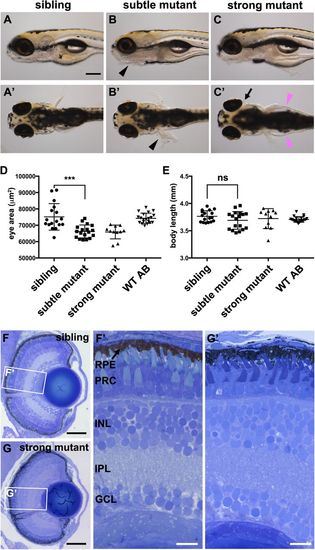
pen/lgl2 larvae have smaller but morphologically normal eyes at 5 dpf. (A–C′) pen/lgl2 eye phenotype at 5 dpf. Panels show examples of sibling (A,A′), subtle mutant (B,B′) and strong mutant (C,C′) phenotypes. Mutants were scored based on skin phenotype (B,B′, black arrowheads). pen/lgl2 mutants with strong phenotype display severe edema (C′, magenta arrowheads) and detachment of the eyes (C′, arrow). Scale bar: 250 µm. (D,E) Measurements of lateral eye surface area (D) and body length (E) at 5 dpf in WT (n=20), pen/lgl2sibling (n=17), subtle (n=18) and strong (n=11) phenotype mutant larvae. pen/lgl2 mutants display a significantly smaller eye size in comparison to sibling larvae. Graphs show mean±s.d. ***P=0.0002; ns, not significant (P=0.1148) by t-test (unpaired, with equal s.d., two-tailed). (F–G′) Toluidine Blue staining of transverse histological sections shows that, in comparison to siblings (F,F′), pen/lgl2 strong mutant retinas laminate normally (G,G′). RPE, retinal pigment epithelium; PRC, photoreceptor cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars: (F,G) 50 µm; (F′,G′) 10 µm. PHENOTYPE:
|
|
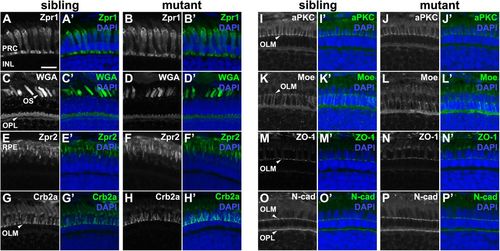
pen/lgl2 mutant distal retina displays no significant abnormalities in polarity or cellular morphology.Immunostaining of transverse retinal sections at 5 dpf. (A,C,E,G,I,K,M,O) Sibling and (B,D,F,H,J,L,N,P) mutant fish. (A–B′) Zpr1, (C–D′) WGA, (E–F′) Zpr2, (G–H′) Crb2a, (I–J′) aPKC, (K–L′) Moe, (M–N′) ZO-1 and (O–P′) N-cadherin immunostaining of distal retina. PRC, photoreceptor layer; INL, inner nuclear layer; OS, outer segment; OPL, outer plexiform layer; RPE, retinal pigment layer; OLM, outer limiting membrane. Scale bar: 10 µm. EXPRESSION / LABELING:
|
|
Lgl2 expression persists in juvenile fish but endogenous zygotic Lgl2 expression is not required for PRC survival.(A–B′) Lgl2 expression in a juvenile [10.6 mm standard length (SL)] retina. (A,A′) Lgl2 expression is detected in the epidermis and the distal retina of juvenile fish. Scale bar: 100 µm. (B,B′) Lgl2 localizes basolaterally in juvenile photoreceptors. RPE expression could not be analyzed due to pigmentation. Scale bar: 20 µm. PRC, photoreceptor cells; OLM, outer limiting membrane; OPL, outer plexiform layer. (C–F′′′) Immunostaining of transverse retinal sections of juvenile chimeric fish. EGFP-labelled sibling pen/lgl2+/+ (C, 5.5 mm SL) or EGFP-labelled mutant pen/lgl2−/− (D, 5.4 mm SL) cells were transplanted into WT hosts and analyzed at 4 weeks. (C,D) Overview of the eye shows location of the transplanted EGFP+ cells in the retina. Boxed regions are shown in E–F′′′. Scale bar: 100 µm. (E–F′′′) Transplanted WT (E–E′′′) or pen/lgl2 mutant (F–F′′′) cells (marked by EGFP) differentiate into various retinal cell types. Different types of cones can be distinguished by cell size. In E″ and F″, arrowheads denote UV cones and MG labels Müller glia processes. White lines in E″ and F″ mark the level of the orthogonal view shown in panels E′′′ and F′′′. Scale bar: 20 µm. EXPRESSION / LABELING:
|
|
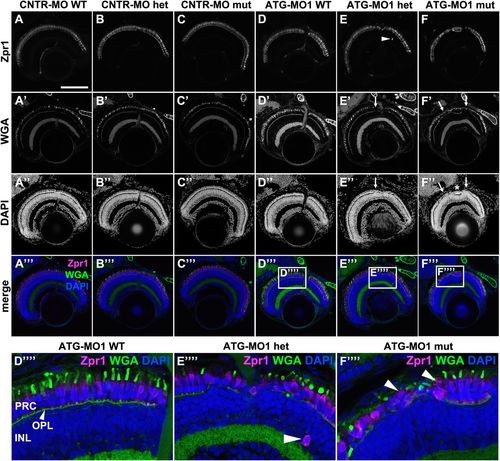
Knockdown of Lgl2 in pen/lgl2 clutches leads to disorganization of retinal lamination. Immunostaining of transverse retinal sections at 5 dpf of MO-injected and genotyped pen/lgl2 clutches. (A–C″′) CNTR-MO-injected fish and (D-F′′′′) ATG-MO1-injected fish. Zpr1 (A–F) stains double cone cell bodies and WGA (A′–F′) the OPL and OSs. In ATG-MO1-injected hetero- (E–E′′′′) or homozygous (F–F′′′′) fish, layering in the distal retina is disturbed. Arrows in E′, E″, F′ and F″ mark disorganized clusters separated by normally aligned cells (asterisk). Boxed areas in D′′′–F′′′ show enlarged views of PRCs in MO-injected pen/lgl2 WT (D′′′′), heterozygote (E′′′′) and mutant (F′′′′) fish. Occasionally Zpr1 positive cells were seen outside of the PRC layer (E,E′′′′, arrowheads). Arrowheads in F′′′′ mark apically displaced PRCs that are localized on top of another PRC. PRC, photoreceptor layer; OPL, outer plexiform layer; INL, inner nuclear layer. Scale bar: 100 µm. |
|
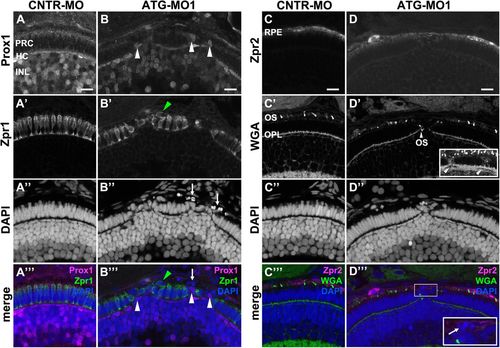
Knockdown of Lgl2 in pen/lgl2 clutches affects the organization of the outer and inner nuclear layers.Immunostaining of transverse retinal sections at 3 dpf of MO-injected pen/lgl2 clutches. (A–A′′′,C–C′′′) pen/lgl2 clutch injected with CNTR-MO and (B–B′′′,D–D′′′) pen/lgl2 clutch injected with ATG-MO1. (A–B′′′) Prox1- (A,B) and Zpr1-(A′,B′) positive cells are well separated from each other in control retinas (A–A′′′) but mix in Lgl2 morphants (B–B′′′). White arrowheads in B and B′′′ denote Prox1-positive cells that have been apically displaced. Green arrowheads in B′ and B′′′ mark an apically displaced PRC. Arrows in B″ and B′′′ mark pyknotic nuclei apical to the photoreceptor layer. (C–D′′′) Zpr2-positive RPE cells (C,D) in MO-injected pen/lgl2 clutches (D–D′′′) surround nuclei apical to the PRC layer. WGA staining (C′,D′) shows that in Lgl2 morphants, disorganized PRCs have outer segments (OS), but these can be found next to the OPL (arrowhead in D′). Projection of multiple optical planes shows breakages in the plane of OPL (inset in D′, arrowheads). Inset in D′′′ shows pyknotic nuclei (arrow) that appear to be surrounded by the Zpr2 signal. PRC, photoreceptor cells; HC, horizontal cells; INL, inner nuclear layer; OPL, outer plexiform layer. Scale bars: 10 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
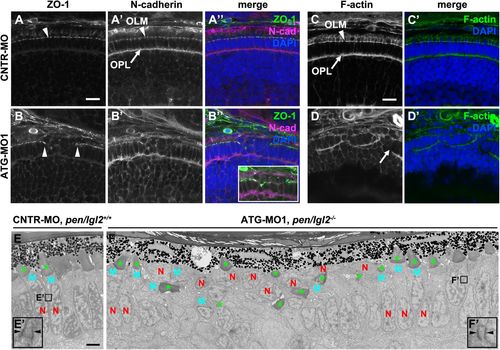
Knockdown of Lgl2 in pen/lgl2 clutches leads to OLM and OPL abnormalities. Immunostaining (A–D′) and TEM (E–F′) of transverse retinal sections at 3 dpf of MO-injected pen/lgl2 clutches. (A–A″, C,C′) pen/lgl2 clutch injected with CNTR-MO and (B–B″,D,D′) pen/lgl2 clutch injected with ATG-MO1. ZO-1 (A,B), N-cadherin (A′,B′) and F-actin (C,D) localization to OLM (arrowheads) and OPL (arrows) is abnormal in morphants. Inset in B″ shows a projection of 15 optical planes in which discontinuity of the OLM is evident. Scale bars: 10 µm. (E,E′) pen/lgl2+/+ sibling injected with CNTR-MO shows a single, aligned layer of PRCs. E′ shows a higher magnification of a PRC adherens junction (arrowheads). (F,F′) pen/lgl2 mutant injected with ATG-MO1. PRCs have differentiated outer segments (green asterisks) but are disorganized. In the cells next to the disorganized area, normal adherens junctions are detected (F′, arrowheads). N, nucleus; M, mitochondria. Scale bar: 2 µm. EXPRESSION / LABELING:
PHENOTYPE:
|