- Title
-
Abrogation of Stem Loop Binding Protein (Slbp) function leads to a failure of cells to transition from proliferation to differentiation, retinal coloboma and midline axon guidance deficits
- Authors
- Turner, K.J., Hoyle, J., Valdivia, L.E., Cerveny, K.L., Hart, W., Mangoli, M., Geisler, R., Rees, M., Houart, C., Poole, R.J., Wilson, S.W., Gestri, G.
- Source
- Full text @ PLoS One
|
The ele mutation is in slbp. (A-A’) Morphology of 2dpf wildtype and ele mutant embryos (red asterisk indicates heart oedema). (B, B’) Heads of 2dpf wildtype (A) and ele (A’) mutant embryos showing the coloboma phenotype in the mutant (arrowhead in B’). (C-C’) RNAseq mapping plot of SNP homozygosity across Chromosome 14 and 15. (D) Domain organisation of wildtype and mutant Slbp predicted proteins. (E) Dot plots of percentages of embryos showing ele phenotypes in control clutches (blue) and in clutches injected with wildtype slbpTT-AA-RFP RNA (red). Each dot is the percentage from one of seven independent experiments. Thick black bars = standard deviation; fine black line = mean. (F) Reverse transcriptase polymerase chain reaction (RT-PCR) for slbp and slbp2 at developmental stages indicated. (G, H) Whole mount RNA in-situ hybridization for slbp (H) and slbp2 (I) at developmental stages indicated. Scale bars: (A, A’, H, I) 250μm; (B and B’) 100μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
slbpty77e mutants have less neurons and show axonal defects. (A-D”) Acetylated α-tubulin labelling of wildtype (A-D) and ele mutant (A’-D”) embryo brains and eyes. Frontal (A-A’; D-D”) and lateral (B- B’) views of brains and lateral views of eyes (C,C’) of 3dpf (A-C’) and 30hpf (D- D”) wildtype (A-D), slbpty77e mutant (A’-D’) and morphant (D”) embryos. Arrowhead in C’ highlights the aberrant extension of RGC axons within the retina. The asterisks in D’-D” highlight aberrantly positioned axons near the midline commissural region in slbp mutant and morphant embryos. (E-E’) Lateral view with anterior to the left of 3dpf wildtype (E) and slbpty77e mutant (E’) embryos showing expression of the Tg(-8.4neurog1:GFP)sb1 transgene (green) labelled neurons and acetylated α-acetylated tubulin labelled axons/neurites (magenta). (F-F’) Frontal views of the telencephalon in 3dpf wildtype (F) and slbpty77e mutant (F’) embryos showing expression of the Tg(lhx5:GFP)b1205 transgene (green) labelled neurons, SV2 labelled neuropil (red) and acetylated α-acetylated tubulin labelled axons/neurites (cyan). Abbreviations: AC, anterior commissure; POC, post-optic commissure; ON, optic nerve; T, telencephalon; PC, posterior commissure; OT, optic tectum; Hb,habenula; E,eye; P,pineal. Scale bars: (A-C”, E, E’) 100μm (D-D”, F, F’) 50μm EXPRESSION / LABELING:
PHENOTYPE:
|
|
Slbp is required cell autonomously for retinal neuron differentiation. (A, A’) Images from live timelapse recordings of Tg(atoh7:GFP)rw021 GFP-transgene expression in retinal neurons in control (A) and slbpty77e (A’) eyes at 30hpf. (B-B’) Frontal sections of 3dpf wildtype (B) and slbpty77e (B’) retinas showing anti-γ-tubulin labelled neurites/neuropil (magenta) and zpr1-expressing cone photoreceptors (green). The arrowhead points to a few remaining zpr1+ cone cells in the slbpty77e mutant eye. (C-C’) Frontal sections of 3dpf wildtype (wt) host retinas containing transplanted wildtype (C) or slbpty77e mutant (C’) GFP-labelled cells (green).(D-D’) High magnification images of transplanted cells in C-C’. Note the break in the plexiform layer in the retina (arrowhead) in the vicinity of the slbpty77e mutant cells. Abbreviations: nr, nasal retina. Scale bars: (A-C’) 50μm; (D-D’) 25μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Suppressing cell death partially restores axonal/neuropil deficits in the tectum of slbpty77e mutants. (A-B’) Lateral view of 26hpf wild type (A,B) and slbpty77e (A’,B’) heads and eyes stained with TUNEL. Apoptosis is prominent in the tectum (arrowhead) and dorsal hindbrain of the mutant (A’) but not within the eye (B’). The white lines outline the lens within which there is apoptosis in both the wildtype and mutant eyes. (C-C”) Lateral view of 72hpf untreated wildtpe (C), untreated slbpty77e (C’) and (C”) caspase inhibitor treated slbpty77e heads labelled with anti-acetylated tubulin (axons, green) and anti-SV2 (neuropil, red). Arrow shows aberrant retinal ganglion axons in the mutant eye. Abbreviations: AC, anterior commisure; E, eye; OT, optic tectum; T, telencephalon. Scale bar: (A-A’,C-C”) 100μm; (B-B’) 50μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
slbpty77e mutant cells fail to transition from proliferation to differentiation. (A,A’) DNA content of 48 hpf wildtype and slbpty77e embryos as assessed by flow cytometry. (B,B’, C,C’) Transverse sections through 48hpf wildtype (B,C) and slbpty77emutant (B’,C’) eyes immunostained with anti-BrdU (B,B’) and anti-pH3 antibodies (C,C’). (D,D’) Series of projections of confocal images extracted from live time-lapse movies of tg(EF1α:mAG-zGem(1/100))rw0410h; tg(EF1α: mKO2-zCdt(1/90))rw0405b transgenic wildtype (D) and slbpty77e mutant (D’) embryos showing cell cycle progression in cells of the forming somites. Green cells are proliferative (S, G2, M) whereas red cells are differentiating somite cells. Scale bar: (B-C’) 50μm; (D-D’) 300μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
RNAseq analysis reveals that loss of Slbp function induces large-scale changes in gene expression levels consistent with many cells failing to transition to differentiation. (A) Volcano plot displaying differential expressed genes between wildtype and slbpty77eembryos The red dots on the right represent the significant up regulated expressed transcripts (p < 0.01, false discovery rate (FDR) q < 0.01); the red dots on the left represent the transcripts with expression significantly down regulated (p < 0.01, FDR q < 0.01). Non-significant genes (q > 0.01) are represented by a black dot. (B) GO term categories for enriched genes. (B’, B”) Pie charts showing percentages of GO terms relating to each category for up (B) and down (B’)-regulated genes in slbpty77e mutants compared to wildtype. C) Graph showing real time PCR quantification of expression changes for genes selected from the RNAseq dataset. Samples were normalized to β-actin and wildtype values for each gene were set to 1. Fold changes in mutants were plotted relative to this value. (D-F’) Lateral views of 3dpf wildtype (D-F) and slbpty77emutant (D’-F’) heads/eyes showing expression of h2afx (D, D’); gnb3a (E,E’) and pcdh10(F,F’). Note that expression changes for h2afx and gnb3a (C) are consistent with qPCR data (yellow box in C). Scale bars: 100μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
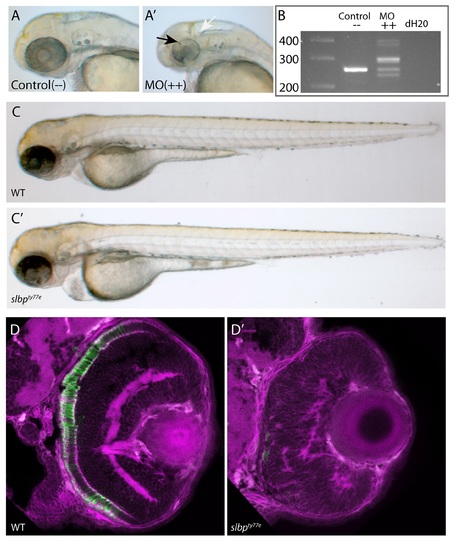
(A-A’) Analysis of live 50hpf Control (A) and Morpholino injected embryos (A’) show that the morphological dent caudal to the MHB (white arrow), indentations in the retina (black arrow) and coloboma are successfully phenocopied. (B) Electrophoresis gel of RT-PCR analysis confirmed that several missplicing events occur as at least five variably sized products were generated (++ lane). (C-C’) Injection of degradation-resistant slbpTT-AA-RFP synthetic RNA into WT(C) and ele(C’)mutants rescues ele phenotype. Note heart oedema still present in some cases (C’). (D-D’) Frontal sections of 3dpf wildtype (D) and slbpty77e (D’) retinas showing anti-γ-tubulin labelled neurites/neuropil (magenta) and Rho4D2-expressing rod photoreceptors (green). (TIF) EXPRESSION / LABELING:
PHENOTYPE:
|
|
Views of heads/brains of wildtype (A-H) and slbpty77e (A’-H’) embryos showing expression of genes indicated to the left of each row. Genotype is indicated at top of each column. Lateral views (A,A’,C,C’,E,E’); dorsal views (B,B’,D,D’,F,F’,G,G’,H,H’). All embryos are 60hpf apart from G,G’ which are 30hpf. Scale bars: 100μm. (TIF) |
|
Images of wildtype (wt, A-F) and slbpty77e (A’-F’) heads (A-C’; E,E’) and eyes (D-F’) at 60hpf showing expression of genes indicated to the left of each row. Genotypes indicated at top of each column. Lateral (A,A’; C-F’) and dorsal view (B,B’). Scale bars: 100μm. (TIF) |

Unillustrated author statements PHENOTYPE:
|