- Title
-
Immune responses in cardiac repair and regeneration: a comparative point of view
- Authors
- Lai, S.L., Marín-Juez, R., Stainier, D.Y.R.
- Source
- Full text @ Cell. Mol. Life Sci.
|
Cardiac injury models. Illustration of myocardial infarction (MI), resection, cryoinjury and genetic ablation of cardiomyocytes (CMs). MI is induced by surgically ligating the left anterior descending coronary artery, leading to tissue death downstream of the ligature. Resection is used to remove part of the ventricle. Cryoinjury is used to cauterize part of the ventricle with a cryoprobe. Genetic ablation is achieved by driving CM-specific Nitroreductase (NTR) expression, which in turn converts a prodrug into a cytotoxic product leading to CM death; alternatively, CM-specific expression of the Diphtheria toxin receptor (DTR) will render the CMs susceptible to diphtheria toxin (DT)-induced cell death |
|
Inflammation induced by cardiac injury. Sterile inflammation can be triggered by various components released by necrotic cells, including DAMPs, proteases, hydrolases and mitochondrial ROS. DAMPs directly activate PRRs on surveillant cells, including tissue macrophages, circulating monocytes and neutrophils, as well as on resident cells, including endothelial cells, fibroblasts and CMs. Proteases, hydrolases and ROS activate the complement system as well as inflammasomes, and degrade the ECM, altogether further propagating the inflammatory response. Activated tissue resident macrophages secrete cytokines to attract monocytes and neutrophils, activate endothelial cells to promote cell adhesion and permeability, and remodel the ECM. Infiltrating monocytes and neutrophils clear cell debris by phagocytosis and help terminate the initial insult. After wound clearance, myofibroblasts secrete ECM to help prevent the injured heart from rupturing. Differentiated Tregs tune down the inflammation by secreting anti-inflammatory cytokines, in parallel with M1–M2 macrophage polarization and programmed neutrophil apoptosis. Inflammation initiation, propagation and resolution can occur in both regenerative and non-regenerative models. However, in the regenerative models, these processes seem to facilitate CM dedifferentiation and proliferation and scar resolution by mechanisms yet to be determined |
|
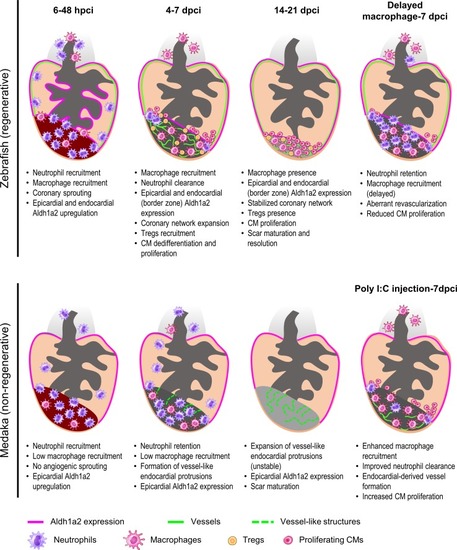
Comparative analyses in zebrafish and medaka after cardiac injury. At 6–48 h post cryoinjury (hpci) in zebrafish, neutrophils and macrophages have been recruited to the damaged tissue, coincident with angiogenic sprouting from existing coronaries and activation of |
|
Comparative analyses in neonatal and adult mice after cardiac injury. In neonatal mice, embryonic macrophages with M2-like properties expand and dominate the injured area, leading to minimal inflammation, angiogenesis, and vigorous CM proliferation. T cells are also prone to differentiate into Tregs at this stage, resolving inflammation and stimulating CM proliferation by secreting mitogens [ |