- Title
-
Endocytic trafficking factor VPS45 is essential for spatial regulation of lens fiber differentiation in zebrafish
- Authors
- Mochizuki, T., Kojima, Y., Nishiwaki, Y., Harakuni, T., Masai, I.
- Source
- Full text @ Development
|
Ectopic lens fiber differentiation occurs in rw341 mutant lens epithelium. (A) Wild-type and rw341 mutant embryos. (B,C) Wild-type (B) and rw341 mutant (C) heads. (D,E) Retinas of wild type (D) and rw341 mutants (E). rw341 mutants show a small lens fiber core, which is surrounded by many aggregated lens cells. (F,G) Higher magnification of the lenses in D and E. (H,H′) Labeling of lenses using anti-Pax6 and PCNA antibodies. In wild type, lens epithelial cells express Pax6 and PCNA (H′, filled arrowheads). Open arrowheads in H′ indicate cells with relatively weak PCNA expression. In multilayered lens epithelium of rw341 mutants (H′, le), most cells express PCNA, but Pax6 expression is weak. (I,I′) Labeling of lenses using anti-Pax6 and BrdU antibodies. Arrows indicate BrdU signals. (J) Percentage of BrdU-positive cells among Pax6-positive lens epithelial cells. Data are mean±s.d. No statistical difference between wild type and rw341 mutants (Student's t-test). (K,K′) Labeling of lenses using anti-Prox1 and PCNA antibodies. In wild type, Prox1 is not expressed in lens epithelium (K′, open arrowheads) and only in early differentiating lens fiber cells (K, arrows). In rw341 mutants, many lens epithelial cells (K′, le) express Prox1 (K′, filled arrowheads). (L,L′) Labeling of lenses using anti-AQP0 antibody. AQP0 is expressed in elongating lens fiber cells in wild type and rw341 mutants (L′, arrows). AQP0-expressing cells were observed in multilayered rw341 mutant lens epithelium (L′, arrowheads). (M) Schematic drawing of wild-type and rw341 mutant lenses. Pax6 is expressed in lens epithelium, whereas Prox1 and AQP0 are expressed in newly differentiating and later elongating lens fiber cells, respectively. In rw341 mutants, ectopic lens fiber differentiation occurs in multilayered lens epithelium. Areas where lens fiber differentiation occurs and does not occur are indicated by red (+) and blue (−), respectively. All lenses are at 5 dpf. Scale bars: 40 µm in B,C; 20 µm in D-L′. |
|
The rw341 mutant gene encodes VPS45. (A) Genomic and cDNA organization of vps45 gene in rw341 mutants. (B) 5 dpf lenses of rw341 mutants (left) and rw341 mutants injected with wild-type (middle) or mutant (right) vps45 mRNA. Wild-type vps45 mRNA rescues multilayer phenotypes (middle, arrowheads). (C) Percentage of transparent lens fiber area in wild type, rw341 mutants and in rw341 mutants injected with wild-type or mutant vps45 mRNA. Wild-type vps45 mRNA significantly increases transparent area size in rw341 mutants. (D) 5 dpf lenses of wild type (left), rw341 mutants (middle) and rw341 mutants expressing VPS45-GFP under control of the foxe3 promoter (right). foxe3 promoter-mediated VPS45-GFP rescues multilayer phenotypes (right, arrowheads). (E) Percentage of transparent lens fiber area in wild type, rw341 mutants and rw341 mutants expressing VPS45-GFP under control of the foxe3 promoter. foxe3 promoter-mediated VPS45-GFP fully recovers transparent area size in rw341 mutants. (F) 5 dpf lenses of wild-type (left), rw341 mutants (middle) and rw341 mutants expressing VPS45-GFP under control of the αA-crystallin promoter (right). Multilayer phenotypes of lens epithelium become milder, but there is still cell aggregation in the posterior lens area in rw341 mutants overexpressing VPS45-GFP under the αA-crystallin promoter (right, asterisk). (G) Percentage of transparent lens fiber area in wild type, rw341 mutants and rw341 mutants expressing VPS45-GFP under control of the αA-crystallin promoter. αA-crystallin promoter-mediated VPS45-GFP partially recovers transparent area size in rw341 mutants, but the recovery does not reach the wild-type level. (H) Prox1 expression (green) in wild type, rw341 mutants and rw341 mutants expressing VPS45-GFP under control of foxe3 promoter or αA-crystallin promoter. Bottom panels indicate higher magnification of outlined areas. Ectopic Prox1 expression in rw341 mutants is inhibited by foxe3 promoter-induced VPS45-GFP (arrowheads), but not by αA-crystallin promoter-induced VPS45-GFP (asterisks). Fluorescent signals of VPS45-GFP were inactivated by the paraffin sectioning process. (C,E,G) Data are mean±s.d. Two-way (C) and one-way (E,G) ANOVA: *P<0.05, ***P<0.005. Scale bars: 20 µm. |
|
Endocytic trafficking defects cause rw341 mutant lens phenotypes. (A) 5 dpf lenses of wild type, rw341 mutants and rw341 mutants injected with rabenosyn 5 mRNA. (B) Percentage of transparent lens fiber area in wild type, rw341 mutants and rw341 mutants injected with vps45 or rabenosyn 5 mRNA. rabenosyn 5 mRNA increased transparent area size in rw341 mutants, although the difference was less significant (P=0.058). (C) 5 dpf lenses of wild type, rw341 mutants and rw341 mutants injected with rab5aa, rab5c or rab11a mRNA. (D) Percentage of transparent lens fiber area in wild type, rw341 mutants and rw341 mutants injected with rab5aa, rab5c or rab11a mRNA. All types of rab mRNA increased transparent area size in rw341 mutants, although rab5aa mRNA injection was not significant. (E) Expression of GFP-tagged rab5c and rab7 in wild-type and rw341 mutant lens epithelium at 48 hpf. (F) Expression of GFP-tagged rab11a in wild-type and rw341 mutant lens epithelium at 48 hpf. The left panel provides a schematic drawing of a lens epithelial cell with a nucleus (blue) and recycling endosomes (green). Two different level confocal images indicate GFP-rab11a signals densely located beneath apical surface membranes, which face the lens fiber core (lf) at the posterior-most position along the AP axis. Right panels indicate three neighboring confocal images of wild-type and rw341 mutant lens epithelium. In wild-type lens, strong patchy GFP-rab11a signals are observed in the apical region of lens epithelial cells (arrows) adjacent to lens fiber core. In contrast, there are no GFP-rab11a signals in rw341 mutants. (G-I) Percentage of GFP-tagged rab5c (G), rab7 (H) and rab11a (I)-positive area relative to total apical area. (B,D,G-I) Data are mean±s.d. (B,D) Two-way ANOVA. (G-I) Student's t-test: *P<0.05, **P<0.01, ***P<0.005. Scale bars: 20 µm. PHENOTYPE:
|
|
Ectopic lens fiber differentiation in rw341 mutants is independent of FGF signaling. (A) Prox1 and PCNA expression in wild-type lenses. DMSO treatment does not affect PCNA and Prox1 expression, which are normally expressed in lens epithelium and newly differentiating lens fiber cells (open arrows), respectively. SU5402 treatment inhibits Prox1 expression. Almost all cells along the posterior margin of the lens fiber region express only PCNA (arrowheads). A few weakly Prox1-expressing cells are observed inside PCNA-positive posterior marginal cells (filled arrows). (B) AQP0 and PCNA expression in wild-type lenses. Bottom panels indicate higher magnification of outlined areas in the upper panels. DMSO treatment does not affect AQP0 expression. A few peripheral PCNA-positive cells express AQP0 (open arrows). In SU5402 treatment, almost all PCNA-positive posterior marginal cells did not express AQP0 (asterisks). (C) Prox1 and PCNA expression in rw341 mutant lenses. DMSO treatment does not change Prox1 expression in the posterior lens fiber region (open arrows) as well as the anterior multilayered epithelium (le). SU5402 treatment also did not inhibit Prox1 expression in either the anterior lens epithelium (le) or the posterior lens fiber region (filled arrows). (D) AQP0 and PCNA expression in rw341 mutant lenses. Bottom panels indicate higher magnification of outlined areas in the upper panels. AQP0-positive cells are detected in the anterior multilayered lens epithelium of both DMSO- and SU5402-treated rw341 mutants (open arrows). (E) Number of Prox1-positive cells in the anterior lens epithelium per lens section. There are no Prox1-positive cells in wild-type lens epithelium treated with either DMSO or SU5402. SU5402 treatment did not reduce Prox1-positive cells in rw341 mutants. (F) Percentage of Prox1-positive cells relative to total lens epithelial cells. SU5402 treatment did not reduce the fraction of Prox1-positive cells in rw341 mutants. (G) Number of Prox1-positive cells in the posterior lens area per lens section. SU5402 treatment drastically reduces Prox1-positive cells in wild type, but did not in rw341 mutants. (E-G) Data are mean±s.d. (E,G) Two-way ANOVA. (F) Student's t-test: *P<0.05, **P<0.01, ***P<0.005. Scale bars: 20 µm (10 µm in higher magnification images in B,D). |
|
Ectopic lens fiber differentiation depends on TGFβ signaling. (A) 5 dpf wild-type and rw341 mutant lenses treated with DMSO and SB505124. In DMSO-treated rw341 mutants, lens epithelial cells form multilayers (asterisk). SB505124 treatment rescues multilayer phenotypes in rw341 mutants (arrowheads). (B) Percentage of transparent lens fiber area in wild type and rw341 mutants treated with DMSO and SB505124. SB505124 treatment significantly increases transparent fiber area size in rw341 mutants, although the recovery does not reach the wild-type level. (C) Prox1 (green) and PCNA (magenta) expression in 5 dpf rw341 mutant lenses treated with DMSO and SB505124. Lower panels indicate higher magnification of lens epithelia (le). lf, lens fiber. Upper right and lower panels show only the green channel. In DMSO-treated rw341 mutants, most cells express Prox1 in multilayered lens epithelium (le). In BS505124-treated rw341 mutants, the number of Prox1-positive cells is markedly reduced (arrowheads). Asterisks indicate the Prox1-negative lens epithelial area. (D) AQP0 (green) and PCNA (magenta) expression in 5 dpf rw341 mutant lenses treated with DMSO and SB505124. Lower panels indicate higher magnification of lens epithelia. Upper right and lower panels show only the green channel. In DMSO-treated rw341 mutants, AQP0-positive lentoid-like structures are observed (arrowheads). In SB505124-treated rw341 mutants, only one PCNA-positive cell expresses AQP0 (arrowhead), but lentoid-like structure is not observed (asterisks). (E) The number of Prox1-positive cells in anterior lens epithelium per lens section. (F) Percentage of Prox1-positive cells relative to the total number of lens epithelial cells. (B,E,F) Data are mean±s.d. (B) Two-way ANOVA. (E,F) Student's t-test: *P<0.05, **P<0.01, ***P<0.005. Scale bars: 20 µm. |
|
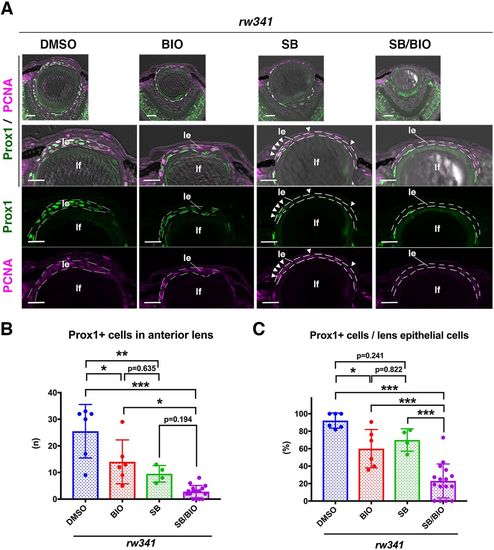
Wnt activation rescues ectopic lens fiber differentiation in rw341 mutants. (A) Prox1 (green) and PCNA (magenta) expression in 5 dpf rw341 mutant lenses treated with DMSO, BIO, SB505124 and SB505124/BIO. The second row provides higher magnification of lens epithelia (le). Third and fourth rows show only green and magenta channels, respectively. In BIO-treated rw341 mutant lenses, lens epithelial cells still form multilayers and express Prox1. In SB505124-treated rw341 mutants, lens epithelium is monolayered and the number of Prox1-positive cells is reduced (arrowheads). In rw341 mutants treated with SB505124 and BIO, lens epithelium is monolayered and almost all lens epithelial cells are Prox1 negative. lf, lens fiber. (B) The number of Prox1-positive cells in lens epithelium of rw341 mutants per lens section. Either BIO or SB505124 treatment reduced the number of Prox1-positive cells. Dual treatment with SB505124 and BIO more effectively reduced the number of Prox1-positive cells in rw341 mutants than BIO treatment. However, no significant difference between SB505124 and BIO/SB505124 treatments may indicate that effective rescue of monolayered epithelial structure by SB505124 is enough to reduce ectopic Prox1 cell number. (C) Percentage of Prox1-positive cells in anterior lens epithelium of rw341 mutants. BIO treatment significantly reduced the percentage, which is similar to the outcome of SB505124 treatment. Dual treatment with SB505124 and BIO more effectively reduced the percentage of Prox1-positive cells than either SB505124 or BIO treatment alone. (B,C) Data are mean±s.d. One-way ANOVA: *P<0.05, **P<0.01, ***P<0.005. Scale bars: 20 µm. |
|
Lens epithelial phenotypes of the rw341 mutant (A) Retinal ciliary marginal zone (CMZ) of wild-type and rw341 mutant embryos at 5 dpf. Retinal stem cells and progenitor cells, which are located in the retinal CMZ, were swollen in rw341 mutants. (B) Labeling of wild-type and rw341 mutant lenses with phalloidin (magenta) and a nuclear stain, Sytox-Green (green) at 3, 4, and 5 dpf. Higher-magnification images of squares in upper panels are shown at the bottom of each panel. In rw341 mutants, lens epithelium is monolayered at 3 dpf, but lens epithelial nuclei become swollen and start to pile up (arrowheads) at 4 dpf. Irregularly aggregated cells (arrows) are observed in the posterior region of the lens in rw341 mutants at 5 dpf. (C) Labeling 5 dpf wild-type and rw341 mutant lenses with anti-ZO1 antibody (green). Upper and lower panels indicate a lens and higher magnification of lens epithelium, respectively. Dot-like ZO1 signals are detected at the apical interface between wild-type lens epithelial cells (open arrowheads), whereas dotted ZO1 signals are irregularly observed in multilayered lens epithelium of rw341 mutants (le). (D) Labeling of 5 dpf wild-type and rw341 mutant lenses with antibodies against E-cadherin (green). Nuclei were stained with TOPRO3 (white). Upper left and right panels indicate an eye and a lens, respectively. Lower panels indicate higher magnification of the lens epithelium area. Right upper and lower panels indicate only the green channel. E-cadherin is normally localized at the adherens junction between wild-type lens epithelial cells (open arrowheads), but scattered in the multilayered lens epithelium of rw341 mutants (le). (E) Labeling of 5 dpf wild-type and rw341 mutant lenses with anti-pH3 (green) and anti-PCNA (magenta) antibodies. Nuclei were counter-stained with TOPRO3 (white). Upper left and right panels indicate an eye and a lens, respectively. Lower panels indicate higher magnification of lens epithelium area. pH3-positive lens epithelial cells are few in wild type (open arrowhead) and do not increase in rw341 mutants. (F) Confocal scanning of 5 dpf wild-type and rw341 mutant lenses with a transgene, Tg[acta2: EGFP] (green). Upper left and right panels indicate an eye and a lens, respectively. Lower panels indicate higher magnification of lens epithelium area. No EGFP expression was observed in either wild-type or rw341 mutant lens epithelium (le). Scale bars: 20 μm. |
|
Lens epithelial cells enter fiber differentiation without passing through the equator in rw341 mutants (A, B) Labeling of wild-type (A) and rw341 mutant (B) lenses with anti-Prox1 antibody. Nuclei were counterstained with TOPRO3. Right panels indicate higher magnification of lens epithelium area shown by squares in the left panels. In rw341 mutants at 3dpf, some cells express Prox1 in monlayered lens epithelium (B, arrowheads) . Prox1-positive cells increase and the monolayer of lens epithelium is disrupted at 4 dpf. (C, D) Prox1 (C) and AQP0 (D) expression in 9 dpf wild-type and rw341 mutant lenses counter-labeled with anti- PCNA antibody. In rw341 mutant lenses, aggregated anterior lens cells are observed close to the transparent lens fiber core. Only a few cells express Prox1 (C arrowheads), and most cells express AQP0 (D, asterisk). AQP0- positive cells are still nucleated, suggesting that denucleation process may be compromised. (E) Developmental profile of lens phenotypes in rw341 mutants. rw341 mutant lens epithelial cells start to express Prox1 at 3 dpf, and then Prox1 expression switches to AQP0 expression after 5 dpf. Most cells express AQP0 to form lentoid at 9 dpf. Scale: 20 μm (A, B). Development: doi:10.1242/dev.170282: Supplementary information Development • Supplementary information |
|
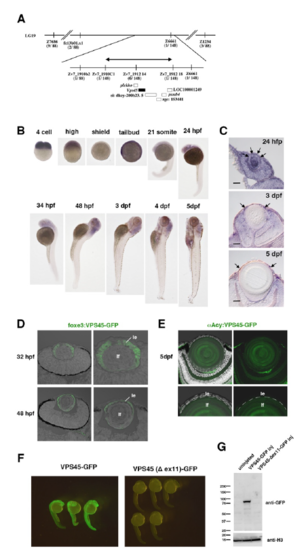
Cloning of rw341 mutant gene (A) The rw341 mutation is mapped on chromosome 19 and is restricted within the genomic region, flanked by two polymorphic markers, Zv7_1912 14 and Zv7_1912 18 (arrow). In this region, six genes including vps45 are annotated. Information regarding polymorphic markers is provided in Table S1. (B) Whole mount in situ hybridization of wild-type embryos with the vps45 mRNA probe during development. (C) Section of wild-type lenses labeled with the vps45 mRNA probe at 24hpf, 3 dpf and 5 dpf. Arrowheads indicate mRNA signals in lens vesicle (24 hpf) and lens epithelium (3 and 5 dpf). (D) GFP expression in lenses of Tg[foxe3:VPS45-GFP] at 32 and 48 hpf. Right panels indicate higher magnification of lenses. GFP is expressed only in lens epithelium (le) but not in lens fiber core (lf). (E) GFP expression in lenses of Tg[αAcy:VPS45-GFP] at 5 dpf. Bottom panels indicate higher magnification of the interface between lens epithelium and lens fiber core. GFP is expressed only in lens fiber core (lf) but not in lens epithelium (le). (F) Embryos injected with mRNA encoding VPS45-GFP and VPS45(Δex11)-GFP. VPS45-GFP expression is maintained, but VPS45(Δex11)-GFP expression disappears at 24 hpf. (G) Western blotting of 24 hpf wild-type embryos, and wild-type embryos injected with vps45-GFP mRNA and vps45(Δex11)-GFP mRNA with anti-GFP antibody. A band of GFP antibody is detected in vps45-GFP mRNA injected embryos, but disappears in vps45(Δex11)-GFP mRNA injected embryos. |
|
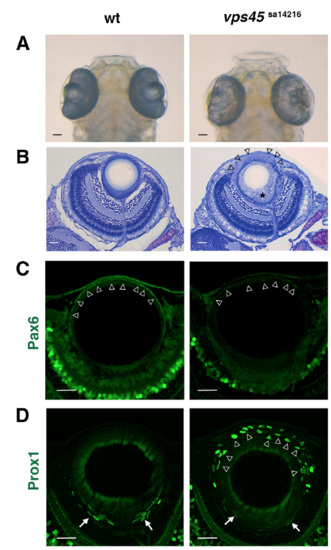
Lens phenotypes of the zebrafish vps45 non-sense mutant allele (A) Heads of wild-type and vps45sa14216 mutants. As in rw341 mutants, a small lens fiber core is observed in the eye cup of vps45sa14216 mutants. (B) Sections of wild-type and vps45sa14216 mutant eyes. Like rw341 mutants, lens epithelium is disrupted to form multiple layers in vps45sa14216 mutants (arrowheads). Suture formation of elongating lens fibers is also abnormal in vps45sa14216 mutants (asterisk). (C) Pax6 expression (green) in wild-type and vps45sa14216 mutant lenses. In wild type, Pax6 is expressed in a monolayer of lens epithelial cells (arrowheads). In vps45sa14216 mutants, Pax6 expression was detected, but becomes weaker than in wild type lenses (arrowheads). (D) Prox1 expression (green) in wild-type and vps45sa14216 mutant lenses. In wild type lenses, Prox1 is expressed only in early differentiating lens fiber cells (arrows). In vps45sa14216 mutants, Prox1 is ectopically expressed in multilayered anterior lens epithelium (arrowheads). Scale bars: 20 μm. |
|
Multilayered lens epithelial phenotypes are not rescued by overexpression of integrin β1a mRNA (A) Lens phenotypes of rw341 mutant embryos injected with integrin β1a mRNA at 5 dpf. Multi-layered phenotypes of rw341 mutants were not rescued by overexpression of integrin β1a mRNA. Scale bars: 20 μm. (B) Percentage of transparent lens fiber area relative to the total lens area in wild-type, rw341 mutants, rw341 mutants injected with vps45 mRNA, and rw341 mutants injected with integrin β1a mRNA. Averages and standard deviations are indicated. Overexpression of vps45 mRNA significantly recovered the transparent area in rw341 mutants. However, overexpression of integrin β1a mRNA did not. Two-way ANOVA, multiple comparison using Turkey: **p<0.01, ***p<0.005. |
|
Extracellular matrix seems to be normal in rw341 mutant lens epithelium (A-D) Electron microscopic analyses of wild-type and rw341 mutant lens at 4 dpf and 5 dpf. Anterior and equatorial regions of lens epithelium are shown. Cornea epithelium (ce), cornea stroma (cs) and cornea endothelium (cn) are indicated in yellow. There is a layer between cornea endothelium and lens epithelium that corresponds to extracellular matrix tissue (red asterisks). In rw341 mutants, this layer is maintained at both 4 and 5 dpf, although three cornea layers are not clearly distinct (c) at 5 dpf. c, cornea; le, lens epithelium; lf, lens fiber cells; n, nucleus; mt, mitochondria. |
|
Pax6 expression in SU5402-treated wild-type and rw341 mutant lenses (A, B) Pax6 and PCNA expression of wild-type (A) and rw341 mutant (B) lenses treated with either DMSO or SU5402. Bottom two large panels indicate higher magnification indicated in squares of the upper panels of SU5402-treated lenses. In DMSO-treated wild type, Pax6 is expressed in lens epithelium, and PCNA is expressed in lens epithelial cells as well as early differentiating lens fiber cells, the latter of which partially overlap with Prox1 expressing cells. In SU5402-treated wild-type lenses, many PCNA-positive cells are located along the posterior margin of the lens fiber region and these cells express Pax6 (A, arrows), suggesting that these PCNA-positive cells maintain lens epithelial cell fate. In DMSO- and SU5402-treated rw341 mutants, Pax6 expression is low in anterior multilayered lens cells. SU5402-treated rw341 mutants, many PCNApositive cells accumulated along the margin of posterior lens fiber core, but, in contrast to SU5402-treated wildtype lenses, these cells did not express Pax6 (B, open arrows). Since these PCNA cells express Prox1 (Fig. 4C), they enter early stage of lens fiber cell differentiation. |
|
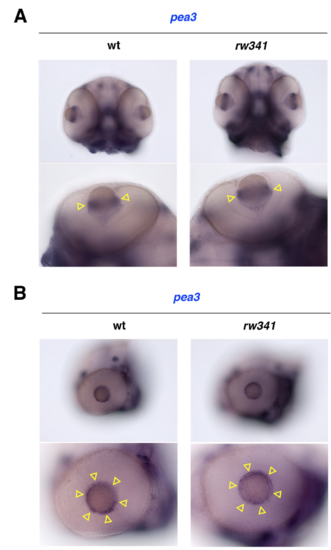
FGF signaling is not altered in rw341 mutant lenses (A, B) Ventral (A) and lateral (B) views of wild-type and rw341 mutant heads labeled with pea3 RNA probe. Lower panels show the eye. pea3 mRNA is expressed along the circumference of the lens equator in wild type (yellow arrowheads). The spatial expression pattern of pea3 mRNA is not changed in rw341 mutants (yellow arrowheads). |
|
SB505124 treatment inhibits TGF-β signaling in zebrafish lens epithelium (A) Labeling of 5 dpf wild-type and rw341 mutant lenses with anti-pSmad2 antibody (magenta) and Sytox Green (green). Upper panels show lens epithelium. Middle panels indicate the magenta channel. Bottom panels show higher magnifications of squares indicated in the upper/middle panels. In wild-type lenses, small dotted pSmad2 signals are observed in lens epithelial cell nuclei (open arrowheads). In rw341 mutants, pSmad2 signals increased throughout the whole region of lens epithelial cell nuclei (filled arrowheads). (B) Labeling of 5 dpf SB505124-treated wild-type and rw341 mutant lenses with anti-pSmad2 antibody (magenta) and Sytox Green (green). Upper panels show lens epithelium. Middle panels indicate the magenta channel. Bottom panels show higher magnification of squares indicated in the upper/middle panels. SB505124 treatment markedly reduces the intensity of pSmad2 signals in both wild type and rw341 mutant lens epithelium, confirming that this concentration of SB505124 effectively inhibits TGF-β signaling in lens epithelium. Scale bars: 20 μm (A, B). |
|
Canonical Wnt signaling activity in lens epithelium is inhibited in rw341 mutants (A) dGFP mRNA expression (blue) in Tg[TOP:dGFP] transgenic wild-type lenses at 2 and 3 dpf. dGFP mRNA is expressed in the peripheral region of lens epithelium (arrows) as well as pigment epithelium associated with retinal CMZ (arrowheads). (B) dGFP protein expression (green) of wild-type and rw341 mutant lenses carrying the transgene Tg[TOP:dGFP] at 5 dpf. Nuclei were counter-stained with TOPRO (white). Middle panels indicate only the green channel. Right panels show higher magnification of the anterior lens area. White dotted lines indicate lens epithelium in wild-type and multilayered anterior lens cells in rw341 mutants. The yellow dotted line indicates the peripheral edge of the posterior lens fiber core. Yellow arrows indicate dGFP signals in early differentiating lens fiber cells in wild-type lenses (middle panels). Very faint dGFP signals were detected in peripheral lens epithelium corresponding to the germinative zone in wild-type lenses (white arrows in the right panels), where dGFP mRNA is expressed (A). There is no dGFP signal in anterior multilayered lens cells (white asterisk) and posterior lens fiber area (yellow asterisk) in rw341 mutants. Scale bars: 20 μm. |
|
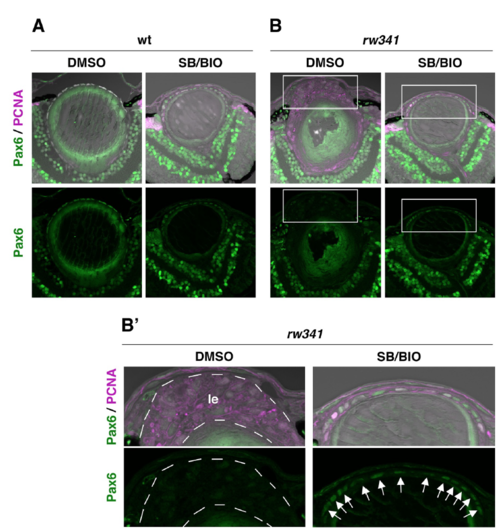
Pax6 expression in SB505124 and BIO-treated wild-type and rw341 mutant lenses (A, B) Pax6 and PCNA expression of wild-type (A) and rw341 mutant (B) lenses. Panels in (B’) indicate higher magnification images of squares in (B), which cover lens epithelium. In DMSO-treated wild type, Pax6 is expressed in lens epithelium. SB505124/BIO-treatment did not affect Pax6 expression in wild-type lenses. In DMSO-treated rw341 mutants, Pax6 expression is low in anterior multilayered lens cells (B’, le). SB505124/BIO-treated rw341 mutants, lens epithelium is monolayered and express Pax6 (B’, arrows). |