- Title
-
sox9b is required in cardiomyocytes for cardiac morphogenesis and function
- Authors
- Gawdzik, J.C., Yue, M.S., Martin, N.R., Elemans, L.M.H., Lanham, K.A., Heideman, W., Rezendes, R., Baker, T.R., Taylor, M.R., Plavicki, J.S.
- Source
- Full text @ Sci. Rep.
|
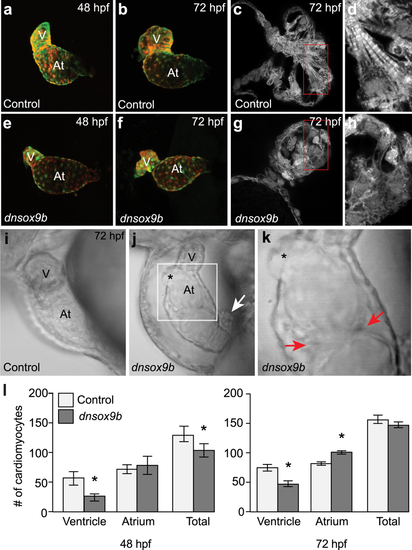
Cardiomyocyte-specific inhibition of sox9b function disrupts cardiac morphogenesis. Live control (a,b and i; Tg(myl7:Gal4VP16;UAS:tRFP)) zebrafish and zebrafish with cardiomyocyte-specific inhibition of sox9b function (e,f,j and k; Tg(myl7:Gal4VP16;UAS:dnsox9b-2A-tRFP)). (a,b,e,f) Embryos and larvae were anesthetized in Tricaine, treated with 20 mM 2,3-butanedione 2-monoxime to temporarily stop heartbeat, then mounted in low melting point agarose for confocal imaging. Ventral images were collected at 48 hpf (a,e) and 72 hpf (b,f). Control (Tg(myl7:Gal4VP16;UAS:tRFP)) embryos had looped hearts and exhibit time dependent chamber growth. Embryos and larvae with cardiomyocyte-specific inhibition of sox9b function (Tg(myl7:Gal4VP16;UAS:dnsox9b-2A-tRFP)) had small, compacted ventricles and unlooped heart chambers. (c,d,g,h) Super-resolution images of fixed control (c and d; Tg(myl7:Gal4VP16;UAS:tRFP)) and experimental larvae (g,h; Tg(myl7:Gal4VP16;UAS:dnsox9b-2A-tRFP)). Cardiomyocyte-specific inhibition of sox9b function disrupted the development of myofibrillar bundles and z-lines were notably absent. (i–k) Still images from Supplemental Movies. (k) Boxed area in j. Pericardial edema, holes in the heart (asterisks in j,k), and ectopic endothelial cushions (red arrows in k) were observed in larvae with cardiomyocyte-specific inhibition of sox9b function. Proepicardial cells were observed adjacent to the myocardium (white arrow in j). (l) Quantification of ventricular and atrial cardiomyocytes at 48 and 72 hpf. Cardiomyocyte-specific inhibition of sox9b function resulted in a decrease in ventricular cardiomyocytes and an increase in atrial cardiomyocytes. Light bars indicate control group and dark bars indicate the experimental group. Asterisks indicate significant differences between groups as determined by Student’s t-test (p < 0.05), n = 8–10 per group. Ventricle (V) and atrium (At) are abbreviated as indicated. PHENOTYPE:
|
|
Cardiomyocyte-specific but not endothelial-specific inhibition of sox9b function results in hypoplastic atrioventricular cushions. Control larvae (a,c,d,d’; Tg(myl7:Gal4VP16;kdrl:GFP)) and larvae with cardiomyocyte-specific loss of sox9b function (b,d,e,e’; Tg(myl7:Gal4VP16;kdrl:GFP;UAS:dnsox9b-2A-tRFP)) were fixed at 80 hpf and processed for fluorescent immunohistochemistry using antibodies against activated leukocyte cell adhesion molecule (Alcam; purple). (a,b) Larvae were mounted ventrally in low melting point agarose and imaged at 40x magnification with a confocal microscope. (c,d) Boxed areas in a & b showing endocardial expressing Alcam, a marker of differentiated endocardial cushion cells. (a,c) AV cushions form normally in control larvae, as indicated by a coalescence of Alcam + endocardial cells at the junction between the atrium and ventricle (boxed area in a). (b,d) Larvae with in cardiomyocyte-specific inhibition of sox9b had hypoplastic cushions with Alcam + endocardial cells. White arrow indicates endocardium pushing through a hole in myocardium. Ventricle (V) and atrium (At) are abbreviated as indicated. Images are representative phenotypes, n = 5 per group. (e-f’) Control larvae (Tg(fli1a:Gal4ff;UAS:Kaede)) and larvae with endothelial-specific loss of sox9b function Tg(fli1a:Gal4ff;UAS:dnsox9b-2A-tRFP; UAS:Kaede)) were fixed at 120 hpf, processed for fluorescent immunohistochemistry using antibodies against Alcam, and scored for the presence of endothelial cushion. Endothelial cushions clearly formed in both control larvae (e) and larvae with endothelial-specific loss of sox9b function (f). Images are representative phenotypes, n = 8 per group. PHENOTYPE:
|
|
Impaired epicardium formation following cardiomyocyte-specific inhibition of sox9b function. Control (Tg(myl7:Gal4VP16;pard3-like:EGFP)) larvae and larvae with cardiomyocyte-specific loss of sox9b function (Tg(myl7:Gal4VP16;pard3-like:EGFP;UAS:dnsox9b-2A-tRFP)) were fixed at 120 hpf and processed for fluorescent immunohistochemistry using antibodies against tRFP (red) and Alcam (blue). Larvae were mounted ventrally in low melting point agarose and imaged at 40x magnification with a confocal microscope. The myocardium is indicated by a dashed line. (a,a’) The epicardium forms normally in control larvae, as indicated by EGFP-labeled epicardial cells on the surface of the ventricular and atrial myocardium (red arrows). (b,b’) Epicardium formation is impaired when sox9b function is lost in cardiomyocytes. Very few EGFP-labeled epicardial cells can be seen on the ventricular myocardium (red arrow) and none are present on the atrial myocardium. A cluster of proepicardial progenitors is visible in the pericardial space (white arrow). (c,d) pard3-like:EGFP zebrafish embryos were injected with either a control plasmid (Tg(myl7:tRFP)) or plasmid with the dnsox9b fused to a cardiomyocyte specific promoter (Tg(myl7:dnsox9b-2A-tRFP)). Injections resulted in mosaic expression of the constructs in cardiomyocytes. Samples were fixed at 96 hpf and processed for immunohistochemistry using an antibody for tRFP (red) and DAPI to label nuclei (blue). With mosaic dnsox9b expression, epicardial cells (red arrows) are found overlying dnSox9b+ myocardial cells. Ventricle (V) and atrium (At) are abbreviated as indicated, and the outflow tract (bulbus arteriousus) is indicated by the yellow arrow. Images are representative phenotypes, n = 8 per group. |
|
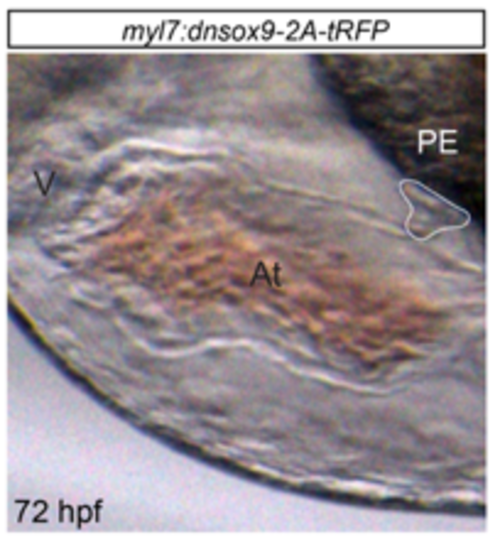
Inhibition of sox9b function in cardiomyocytes does not alter the development of proepicardial progenitor cells. Proepicardium development was examined in control (Tg(myl7:Gal4VP16;UAS:tRFP)) larvae and larvae with cardiomyocyte-specific loss of sox9b expression (Tg(myl7:Gal4VP16;UAS:dnsox9b-2A-tRFP)). 72 hpf larvae were mounted in methylcellulose and imaged using camera mounted on a stereomicroscope. The proepicardium formed in all zebrafish larvae examined (n=7) and can be seen in the above image, outlined in white. Ventricle (V), Atrium (At) and Proepicardium (PE) are abbreviated as indicated. Anterior to the left. |