- Title
-
CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury
- Authors
- Yakulov, T.A., Todkar, A.P., Slanchev, K., Wiegel, J., Bona, A., Groß, M., Scholz, A., Hess, I., Wurditsch, A., Grahammer, F., Huber, T.B., Lecaudey, V., Bork, T., Hochrein, J., Boerries, M., Leenders, J., de Tullio, P., Jouret, F., Kramer-Zucker, A., Walz, G.
- Source
- Full text @ Nat. Commun.
|
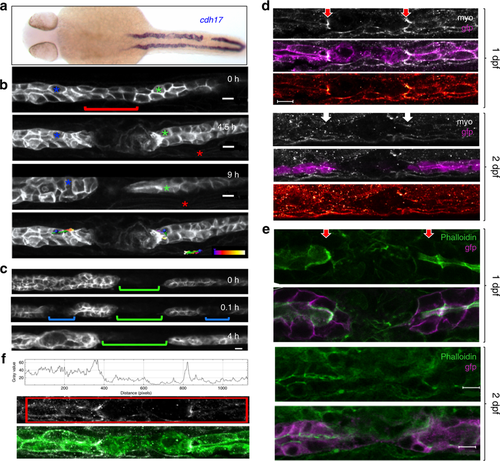
Pronephric ducts injured 1 day after fertilization fail to repair. a In situ hybridization for the pronephros-specific probe cadherin17 (cadh17) reveals that an injury applied 24 h after fertilization (24 hpf) is not repaired. The in situ hybridization was performed 24 h after injury. b Frames taken from a time-lapse movie of cldn2b:lyn-GFP transgenic embryo injured 30 hpf. Cells (blue and green asterisks) next to the gap exhibit little net movement. The gap persisted for 9 h after the injury. The red asterisk marks a somite border serving as a landmark. Tracking lines are color coded, ranging from blue to white (0–9 h) (scale bars, 10 µm). c Repair occurs in the absence of fluid flow. Frames were taken from a time-lapse movie injured first 1 day post fertilization (dpf) (green bracket), with two subsequent injuries (blue brackets) applied 2 dpf. Despite the lack of fluid flow both secondary injuries (blue brackets) recovered after 4 h. d Staining of cldn2b:lyn-GFP transgenic zebrafish embryos wounded at 1 or 2 dpf, fixed 30 min post wounding, and stained for phospho-myosin light chain-2 (MyoP). Activated myosin was detected at the edges of the pronephros injury in 1-day old embryos (red arrows), but not in 2-day-old embryos (white arrows). The bottom panel of each image shows the phospho-myosin levels using a color-coded range indicator (scale bars, 100 µm). e Staining of cldn2b:lyn-GFP transgenic zebrafish embryos wounded 1 or 2 dpf, fixed 4 h after wounding, and stained with phalloidin for actin. Actin staining was detected on the apical surface of cells close to the injury in both 1-day and 2-day old embryos. Actin staining was more prominent in 1-day embryos, and appeared perpendicular to the duct lumen (red arrows) (scale bars, 100 µm). f Quantification of the pixel intensity within the marked region of a single representative confocal plane from the MyoP staining (middle panel) of an embryo injured 1 dpf. The histogram depicts two peaks with increased levels of phosphorylated myosin adjacent to the injury. The bottom panel represents the merged image of GFP/MyoP staining |
|
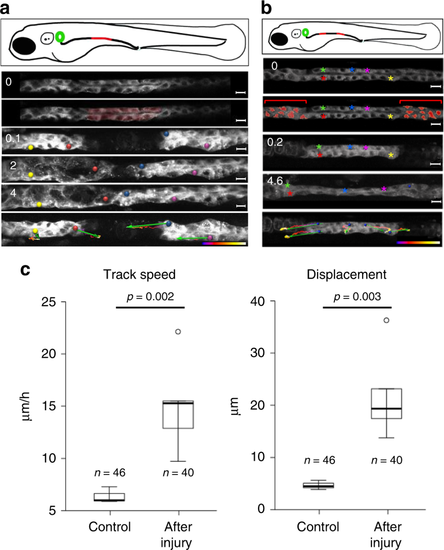
Pronephric duct injuries are repaired by migration. a Frames from Supplementary Movie 1, showing recovery after ablation of a pronephric duct fragment (ablated cells are marked in red). The free ends of the duct move towards each other, whereby the leading cells extend protrusions and exhibit active cell migration. The bottom panel depicts tracking of individual cells over time. Tracking lines are color coded, ranging from blue to white (0–9 h); the green arrows summarize migration direction and cell displacement (scale bars, 10 µm). b Frames from Supplementary Movie 2. The panel depicts a two-sided cell ablation (red-labeled cells), isolating a patch of pronephric cells. The free ends of the isolated duct migrated in opposite direction, stretching the isolated segment. Whereas the cells in the terminal regions exhibited prominent net displacement, the cells in the middle segment remained almost stationary. Tracking lines are color coded, ranging from blue to white (0–9 h) (scale bars, 10 µm). c To compare repair response and collective cell migration, 2-day-old Tg(-8.0cldnb:LY-EGFP), TgBAC(cxcr4b:h2b-RFP) transgenic zebrafish embryos were mounted on the side to image pronephric ducts by confocal microscopy. The pronephros was injured with a two-photon laser at 36 h, and track speed (left panel) (p = 0.0023, t-test) and track displacement length (right panel) (p = 0.0034, t-test) was measured over 2 h. After injury, tubular epithelial cells adjacent to the injury almost tripled their speed. The cell number (n) was derived from three control and six injured pronephric tubules |
|
cxcr4b or cxcl12a deletion does not affect pronephros cell migration. a Collective cell migration in the zebrafish pronephros was quantified, using a cxcr4b:H2B-RFP; cldnb:GFP double transgenic zebrafish line (upper panel, see Supplementary Movie 4). The transgene cxcr4b:H2B-RFP labels the nuclei of cells that express RFP under the control of the endogenous cxcr4b promoter; cldnb:GFP labels the membranes of pronephric duct cells. The pronephros and the corpuscle of Stannius are outlined with dashed lines. The arrow points to the corpuscle of Stannius (scale bars, 10 µm). A model of collective cell migration was generated (lower panel), using the image tool Imaris. The migration of ca. 40 pronephric nuclei was tracked over 8 h, starting 48 hpf. The tracks for each cell were calculated and represented as lines. The observation times were coded by blue-to-green colors; blue are early time points and green are later ones (see Supplementary Movie 5). b The mean track speed and displacement length, i.e., the length that a cell traveled over the observation period were measured at 36 and 41 hpf for 3 h, and at 48 hpf for 8 h. Deficiency of cxcr4b (left panel) or cxcl12a (right panel) did not affect track speed (left panel). c Deficiency of cxcr4b (left panel) or cxcl12a (right panel) did not affect displacement length. Note that the displacement length is larger in the 48–56-hour-interval, because cells were tracked for a longer time period (8 h) in comparison with the earlier time points (3 h each). In contrast, the speed did not change significantly over time (n.s., not significant; t-test) |
|
Defective repair in cxcl12a (−/−) and cxcr4b (−/−) zebrafish embryos. a While control zebrafish embryos repair a lased-induced injury, more than 60% of cxcr4b-deficient zebrafish embryos (means ± SEM; ***p < 0.001; t-test) and b more than 40% of cxcl12a-deficient zebrafish embryos did not repair the wound, but instead revealed various abnormalities (means ± SEM; **p < 0.01; t-test). c The pronephros of 2-day old wild-type cldnb:GFP control zebrafish embryos rapidly regenerates after a laser-induced injury. The arrow points to the regenerated area at 24 h after the injury. The arrowhead points to the cloaca. The pronephros is outlined with dashed lines (scale bar, 100 µm). d Magnification of the regenerating region depicted in c (scale bar, 20 µm). e cxcl12a (−/−) zebrafish embryos fail to regenerate and the pronephric tubule remains discontinued. The arrow points to the gap in the tubule. The pronephros is outlined with discontinued lines. The arrowhead points to the cloaca. Note the dilation of the anterior duct due to ongoing filtration (scale bar, 100 µm). f Magnification of the region that failed to regenerate depicted in d (scale bar, 20 µm). g In some cases, cxcl12a mutant embryos showed aberrant regeneration and formed dorsal pouches. The arrow points to the abnormally regenerated tubule. The pronephros is outlined with discontinued lines. The arrowhead points to the cloaca (scale bar, 100 µm). h Magnification of the abnormally regenerated region depicted in e (scale bar, 20 µm). i cxcr4b mutant zebrafish embryos failed to re-establish the tubular patency similar to cxcl12a mutant embryos. The arrow points to the gap in the tubule. The pronephros is outlined with discontinued lines. The arrowhead points to the cloaca (scale bar, 100 µm). j Magnification of the region that failed to regenerate depicted in f (scale bar, 20 µm) |
|
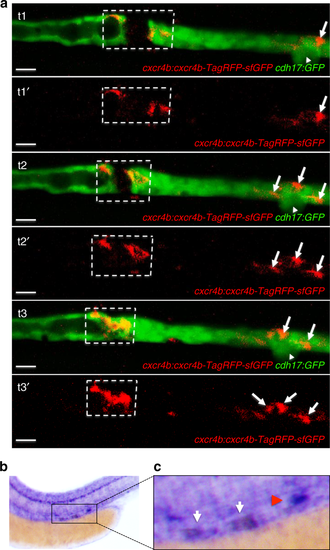
Cxcr4b expression in response to injury. a The GFP-RFP-tagged Cxcr4b (cxcr4b:cxcr4b-tFT) was up-regulated in cells adjacent to the laser-induced injury. The arrowhead points to the corpuscle of Stannius. Arrows point to RFP-positive cells, accumulating distally after the injury. Sequential frames were taken from a time-lapse video at t1 = 96 min, t2 = 120 min, and t3 = 160 min after injury (scale bar, 20 µm). b In situ hybridization revealed up-regulated cxcr4b expression in the leading ends of the regenerating tubule 2 h after laser-induced ablation. The cxcr4b up-regulation is outlined in a dashed box. Note that cxcr4b is strongly expressed in the corpuscle of Stannius (arrow) and in the lateral line. c Magnification of the injury region depicted in b. The white arrows point towards the up-regulated cxcr4b, the red arrowhead towards the corpuscle of Stannius |
|
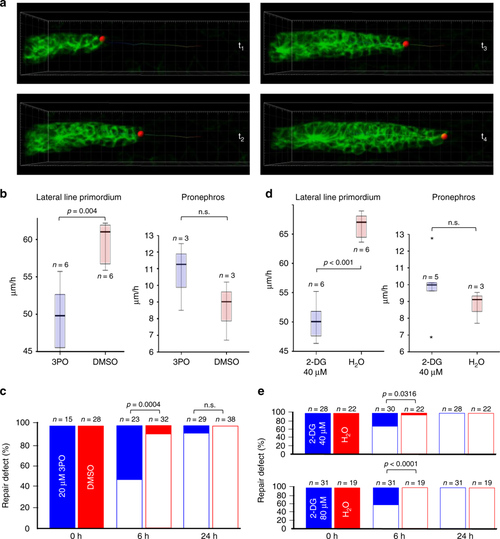
Inhibition of glycolysis slows migration. a Depicted are frames from Supplementary Movie 10, demonstrating the analysis of migration speed in the posterior Lateral Line Primordium (pLLP). A similar imaging approach was used to determine the speed of migrating cells in the zebrafish pronephros. b The small molecule 3-(3-pyri-dinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) at concentrations that had no recognizable developmental effects (20 µM) delayed pLLP migration, but did not affect pronephros cell migration. The box plots represent the median, the first and third quartile (boxed area), and 1.5× interquartile range (whiskers) (n.s., not significant; t-test). c 3PO delayed the repair of a pronephros injury determined at 6 h, but no effect was observed 24 h after the injury (Fisher’s exact test). d The glucose analog 2-deoxy-D-glucose (2-DG) at 40 µM slowed pLLP migration similar to 3PO, but had no effect on pronephros collective cell migration. The box plots represent the median, the first and third quartile (boxed area), and 1.5× interquartile range (whiskers) (n.s., not significant; t-test). e While almost all injuries were repaired 6 h after wounding of control embryos, 40 µM 2-DG reduced the number of repaired injuries to 70% and 80 µM 2-DG to 58%. At 24 h after wounding, all injuries were repaired. Full bars depict not repaired injuries, open bars repaired injuries. The graphs represent the summary of at least three independent experiments (Fisher’s exact test) PHENOTYPE:
|
|
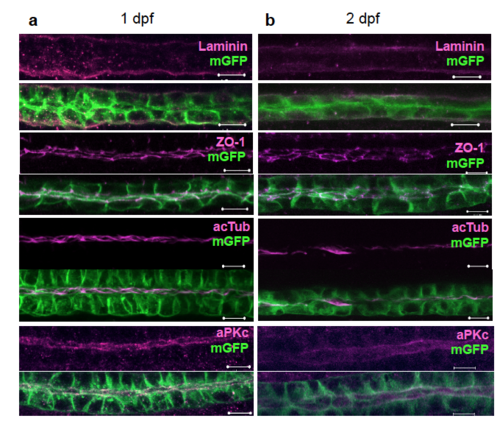
No apparent differences between the zebrafish pronephros at one and two days post fertilization. The pronephric tubules were morphologically similar in one-day- (a) and two-day- (b) old zebrafish embryos. Antibody staining for components of the extracellular matrix (laminin), tight junction complex (ZO-1), cilia (acetylated tubulin), and aPKC did not reveal morphological differences (Scale bars, 10 μm). |
|
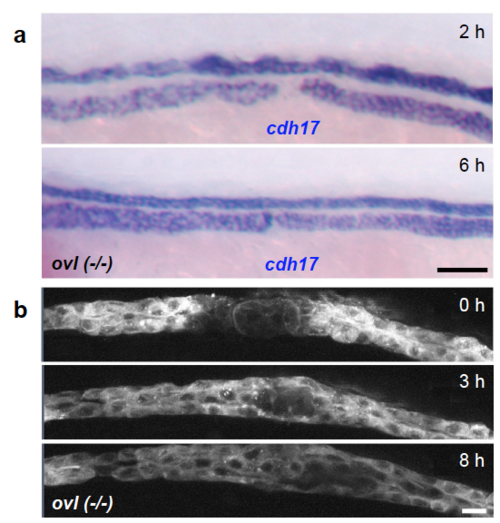
Cilia and fluid flow are not required for pronephros repair (a) Homozygous ift88(-/-) (ovl) zebrafish embryos were injured 3 days after fertilization, and fixed 2 and 6 hours after wounding. In situ hybridization was performed with the pronephros-specific marker cadherin17 (cdh17). At this stage, the embryos displayed prominent cysts and duct dilatations due to ciliary defects. The gap, clearly visible at 2 hours, was repaired 6 hours after injury. The results were confirmed in three independent experiments with at least 3 control and 3 mutant embryos (Scale bar, 100 μm). (b) Frames from a timelapse movie following the recovery in ift88(-/-) (ovl) embryo. The laser-induced injury was completely repaired over an 8-hour time course (Scale bar, 10 μm). |
|
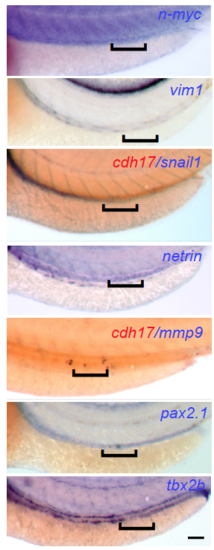
Pronephros repair is independent of EMT. In situ hybridization for genes characteristic for epithelial-to-mesenchymal transition (EMT) or de-differentiation. Two-day-old embryos were injured, fixed 4 hours after wounding, and stained for EMT-typical genes (N-myc, vimentin, snail, netrin and mmp9), or for genes characteristic for non-differentiated pronephric cells (pax2.1 and tbx2b). None of the markers showed up-regulation in the repair region, outlined by a black bracket. The staining for mmp9 and snail was performed in combination with staining for cdh17 labeling the pronephric cells in red (Scale bar, 100 μm). |
|
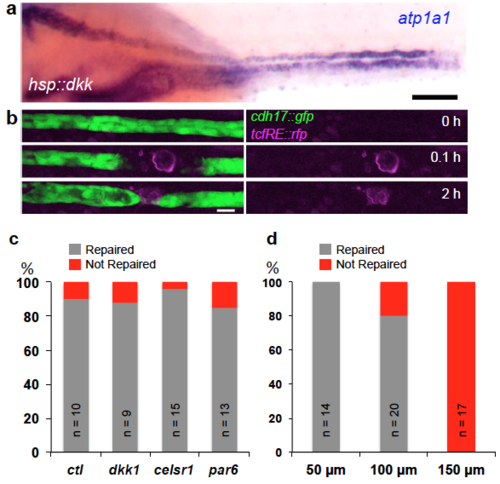
Pronephros repair is independent of Wnt, Celsr1 and Par6 signaling. (a) In situ hybridization with the pronephros specific marker atp1a1a.4, staining injured zebrafish embryo over-expressing the Wnt inhibitor dkk1. The pronephros was injured 2 days after fertilization and fixed 16 hours later. The injured proximal side (arrow) appears dilated, but recovery was not affected by dkk1 over-expression (Scale bar, 100 μm). (b) Frames from a time-lapse movie of injured transgenic embryo carrying a TCF response element, driving the expression of Red Fluorescence Protein (RFP). Laser ablation caused some background fluorescence (red channel) in the injured cells, but no specific RFP expression was detectable in neighboring cells displaying a migratory response (Scale bar, 10 μm). (c) Quantification of the repair process in HSP:dkk1, celsr1 morpholino oligonucleotide (MO) and par6 MO injected embryos that were injured 2 days after fertilization and fixed 16 hours post wounding. While MO-injected embryos displayed duct abnormalities, repair of the pronephros injury was not affected. (d) Injuries ≤ 50 μm in length were repaired in 100%, injuries ≤ 100 μm, corresponding to 10-12 cell diameters, were repaired in 80%, while no injuries ≤ 150 μm were repaired. |
|
Transcriptional profiles of one- and two-day-old zebrafish pronephric tubules. (a) Fluorescently labeled pronephric tubules, isolated from cadh17:gfp transgenic zebrafish embryos. (b) 3D representation of the changes in the total transcript levels between samples from one- (red) and two-day-old zebrafish embryos (blue). Due to technical problems, one of the samples from the first group failed to cluster with the others probes, and was excluded from further analysis. (c) Density histogram representing the distribution of the log2-fold change of all transcripts. The horizontal red lines mark the two-fold change limit. (d) Biological process Gene Ontology (GO) terms enrichment analysis of the transcripts that were up-regulated more than two-fold in the pronephros on the second day of development. Semantically similar categories are represented with similar colors. The size of each box reflects the significance of enrichment (-log10 p-value). The GO analysis was performed with AmiGO and the GO enrichment was graphically represented with REViGO. (e) Comparison with the ZFIN Database and expression profiles, focusing on genes that are specifically expressed in the zebrafish pronephros at 42-60 hours post fertilization, revealed cxcl12a, myca and mafba as potential candidates involved in pronephros repair. |
|
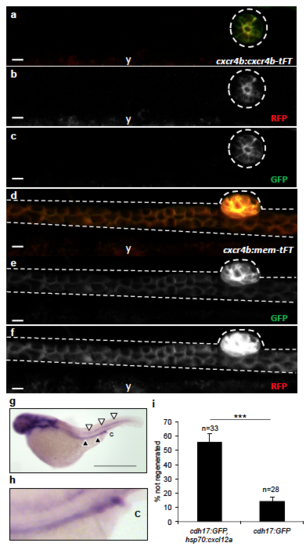
Low-level expression of cxcr4b in pronephric duct cells during normal collective cell migration. (a-c) Using the cxcr4b:cxcr4b-TFT (GFP = short life-time, RFP = long life-time) zebrafish line, lifetime Cxcr4b was only detectable in the corpuscle of Stannius (outlined) at 48 hpf, but not in the pronephros. (d-f) The membrane-resident version cxcr4b:mem-TFT, which does not respond to Cxcl12a signaling and therefore accumulates at the plasma membrane, was detectable in the pronephros (y, yolk) (Scale bars, 10 μm). (g) In situ hybridization depicts expression of cxcl12a in the posterior lateral line (white arrowheads) and in the pronephros (black arrowheads) in zebrafish embryos at 2 dpf (Scale bar, 800 μm). A magnification of the posterior pronephric duct is shown in (h) (c, cloaca). (i) Ectopic expression of Cxcl12a, triggered by heat-shock in the double transgenic cdh17:GFP; hsp-70:cxcl12a zebrafish line, significantly reduces the ability to repair a pronephros injury (means ± SEM; ***, p < 0.001; t-test) . PHENOTYPE:
|
|
myca expression in zebrafish embryos at 1-3 dpf, and up-regulation of myca in response to injury. (a) myca was detected by in situ hybridization at 24 hpf in the zebrafish pronephros. Insert shows the posterior pronephros region close to the cloaca (c, cloaca). myca was strongly expressed in the head, the myotomes (arrow) and in the cloaca (magnification, c) at this stage. (b) Expression of myca was restricted to the head, the somatic furrow (arrow) and the pronephros (arrowheads) at 48 hpf. (c) Expression of myca in the somitic furrows declined at 72 hpf, but remained stable in the head, pronephros (arrowheads) and in the cloaca (c). (d) myca was up-regulated in the pronephric duct cells adjacent to a laser-induced wound at 48 hpf (magnification; c, cloaca) (Scale bars, 50 μm). EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
Zebrafish myca depletion causes a repair defect characterized by abnormal migratory behavior. (a) Single frames taken from the Supplementary Movie 8. Depletion of myca prevented the reversal of the posterior-to-anterior cell migration at the proximal side of the injury, resulting in a failure to repair the injury. (b) Single frames taken from the Supplementary Movie 9. Depletion of myca prevented the repair within 8 hours after a laser-induced injury. Instead of rapidly repairing the injury by a migratory response, the myca-deficient pronephric duct cells remained stationary. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|