- Title
-
An In Vivo Zebrafish Model for Interrogating ROS-Mediated Pancreatic β-Cell Injury, Response, and Prevention.
- Authors
- Kulkarni, A.A., Conteh, A.M., Sorrell, C.A., Mirmira, A., Tersey, S.A., Mirmira, R.G., Linnemann, A.K., Anderson, R.M.
- Source
- Full text @ Oxid Med Cell Longev
|
Time-dependent metronidazole induction of |
|
Metronidazole induces ROS generation in a dose-dependent manner. (a) Representative image of vehicle-treated zebrafish islets ( |
|
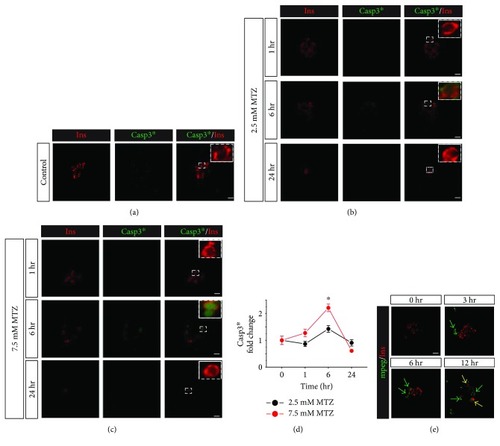
Metronidazole induces apoptosis signaling in |
|
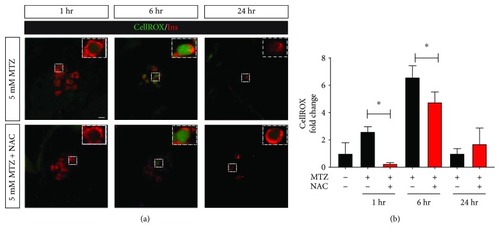
Antioxidant treatment protects from metronidazole-induced ROS generation in |
|
Proposed mechanism of metronidazole-nitroreductase-mediated cell ablation. In the aerobic setting of NTR-expressing eukaryotic cells, we propose that MTZ is reduced to a nitroradical anion by electron transfer from NADH, in a type 2-like mechanism. This radical may be cytotoxic and directly induces DNA damage and apoptosis. Alternately, this radical may regenerate back to metronidazole by electron transfer to O2, concurrently forming superoxide anion and ROS derivatives. This, in turn, drives increased cellular-oxidative stress and triggering of regulated cell death. |