- Title
-
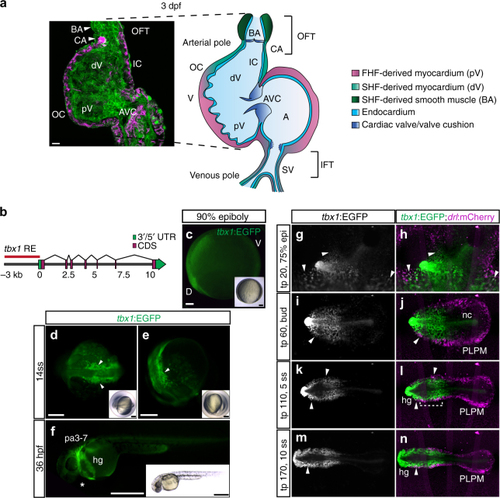
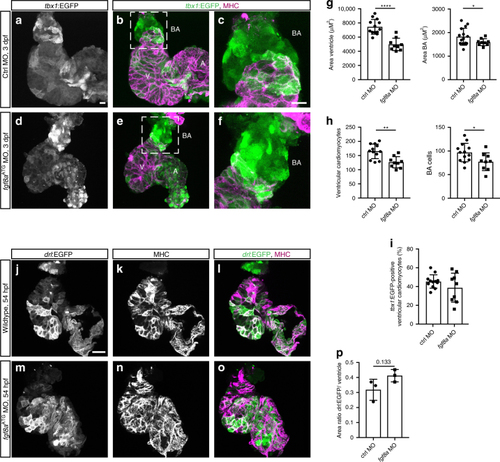
Continuous addition of progenitors forms the cardiac ventricle in zebrafish
- Authors
- Felker, A., Prummel, K.D., Merks, A.M., Mickoleit, M., Brombacher, E.C., Huisken, J., Panáková, D., Mosimann, C.
- Source
- Full text @ Nat. Commun.
|
|
|
|
|
A |
|
|
|
The |
|
|
|
FGF signaling differentially affects |