|
Four cAMP effector proteins are expressed in the heart. Norepinephrine (NE) secreted by sympathetic neurons binds to the β-adrenergic receptor leading to Gs activation and synthesis of cAMP by adenylate cyclase (AC). Acetylcholine (ACh) is secreted by parasympathetic neurons and binds to muscarinergic ACh receptors leading to an activation of Gi causing AC inhibition. The balance of sympathetic and parasympathetic input therefore determines the level of cAMP production. cAMP production in cells is compartmentalized and this is mainly achieved by phoshodiesterases (PDE), which limits cAMP diffusion through degradation. Four effector proteins sense cAMP levels. The best-characterized effector protein is protein kinase A (PKA), which plays a role in cardiac pacemaking, excitation/contraction coupling and cardiac metabolism. The exchange factor directly activated by cAMP (EPAC) has been linked to cardiac hypertrophy, Ca2+-signaling and apoptosis. Often, PKA and EPAC are bound by the same anchor protein (AKAP) along with protein substrates and other enzymes. The hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are important for cardiac pacemaking and ventricular repolarization. Finally, the Popeye domain containing (POPDC) proteins are the most recently identified class of effector proteins and important for cardiac pacemaking, the survival of cardiac myocytes after ischemia/reperfusion and membrane trafficking. |
|
Structure of the Popeye domain containing proteins. ( |
|
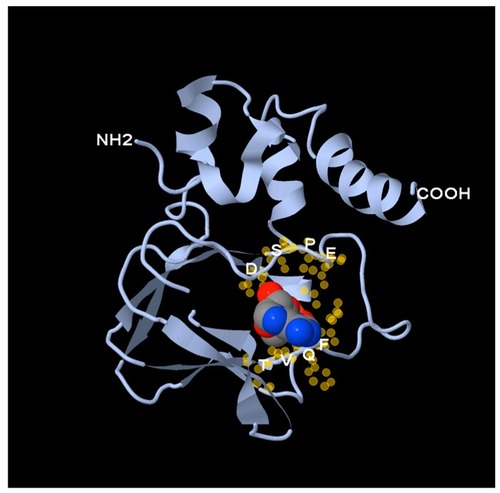
Structure of the Popeye domain. A homology-based structural model of human POPDC1 was generated. A space-filling model of cAMP was placed in the putative phosphate-binding cassette. The DSPE and FQVT motifs, depicted as yellow halos, surround the cAMP molecule and as mutagenesis studies suggest, seem to be directly involved in cAMP-binding. |
|
Expression of POPDC genes in the human body. RNA seq data of |
|
Expression of POPDC1 and POPDC2 in cardiac myocytes. Isolated adult cardiac myocytes were immunostained with ( |