- Title
-
Morphogenetic defects underlie Superior Coloboma, a newly identified closure disorder of the dorsal eye
- Authors
- Hocking, J.C., Famulski, J.K., Yoon, K.H., Widen, S.A., Bernstein, C.S., Koch, S., Weiss, O., FORGE Canada Consortium, Agarwala, S., Inbal, A., Lehmann, O.J., Waskiewicz, A.J.
- Source
- Full text @ PLoS Genet.
|
The superior ocular sulcus in zebrafish, chick and mouse. (A) Zebrafish eyes displaying superior ocular sulci (SOS) marked by an asterisk or arrows. Top row: lateral view DIC image of the eye of a live embryo, photographed on a compound microscope. Enlarged view is shown in panel on right. Bottom row: Left, lateral view surface projection of the eye of a live Tg(rx3:GFP) embryo; Right, surface projection dorsal views of eyes from a Tg(rx3:GFP) embryo. (B) Scanning electron micrographs showing SOS at narrow (top row) and wide (bottom row) phases. Red boxes denote regions enlarged in panels on the right. (C) Single optical section, lateral view, through the eye of an embryo injected with eGFP-CAAX mRNA to label the cell membranes, with right panel showing enlarged view of boxed area. (D) Single optical section, lateral view, through eye of Tg(rx3:GFP) embryo (cyan) immunolabelled for Laminin to highlight the basal lamina (magenta). (E) Diagram showing chick eye with red line demonstrating the plane of section employed on the right. Representative horizontal section through the dorsal eye of a HH16 chick, stained with a Laminin antibody (green) and DAPI (blue). A dorsal, Laminin-lined space is evident in the distal portion of optic cup (asterisk). (F) Diagram showing 3D model of an embryonic eye with red line demonstrating plane of section for both mouse and chick sections. Right three panels are a representative horizontal section through the dorsal eye of an embryonic day 10.5 (E10.5) mouse, stained with a Laminin antibody (green) and DAPI (blue). A dorsal, Laminin-lined space is evident in the distal portion of optic cup (asterisk). Except where noted, scale bars are 50 μm. cf, choroid fissure; D-V, dorsal-ventral; HH, Hamburger Hamilton embryonic stage; hpf, hours post fertilization; N-T, nasal-temporal; nr, neural retina; Pr-Di, proximal-distal. EXPRESSION / LABELING:
|
|
The role of BMPR1 signaling in closure of the superior ocular sulcus. (A-B) Effect of Bmpr1 antagonist DMH1 on SOS closure. Lateral view DIC images of eyes from live embryos (first row) and single optical slices of eyes processed for anti-Laminin immunofluorescence (second row) following exposure to control media or 0.02 μM DMH1, starting at either 10 or 18 hpf (A). SOS is marked by red asterisk. Quantification of delayed sulcus closure in DMH1-treated embryos (B). N = 3 experiments, n = 89 or 90 embryos for each condition. Data are means ± SEM. Statistics is a one-way ANOVA for each time series with Tukey's post-hoc test: **P<0.01. (C) Injection of caBMPR1A mRNA into one-cell stage zebrafish embryos caused expansion of eve1 gene expression into a circular ring in whole embryos at 50% epiboly (5.3 hpf). Significantly fewer embryos exhibited circular eve1 expression when injected with R471H-caBMPR1A. N = 3 experiments. Data are means ± SEM. Statistics is a two-tailed t test: *P<0.05. Scale bars are 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The role of Gdf6a signaling in superior ocular sulcus morphogenesis. (A) Delayed SOS closure caused by Gdf6a knockdown. Tg(rx3:GFP) zebrafish eyes (cyan) from uninjected and Gdf6a morpholino-injected embryos shown as DIC images of live embryos and single optical slices following anti-Laminin antibody staining (magenta). SOS marked by red asterisk. (B) Quantification of embryos with delayed sulcus closure, as assessed at 28 hpf. (C) Time series of maximum projection confocal images of a Tg(rx3:GFP) embryo injected with gdf6a morpholino. (D) DIC images of wildtype, gdf6a+/- and gdf6a-/- eyes (SOS marked by red asterisk). Bottom right panel shows SEM image of a Gdf6a-deficient eye with a pronounced sulcus. (E) Quantification of gdf6a-/- mutants (or siblings) with delayed SOS closure. (F) Adult wildtype zebrafish (top panel) showing normal eye morphology and a gdf6a-/- zebrafish (bottom panel) with superior coloboma (red arrow). N = 3 experiments for graphs in B and E. n = number of embryos. Data are means ± SEM. Statistics in B is a two-tailed t test, and in E is one-way ANOVA with Tukey’s test: **P<0.01, *** P<0.001. Scale bars are 50 μm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Analysis of Tbx2b and closure of the superior ocular sulcus. (A) Whole-mount in situ hybridization for zebrafish tbx2b in control and BMP-depleted embryos. Top panels are eyes dissected from control and DMH1-treated embryos; bottom panels are from gdf6a+/+, and gdf6a-/- embryos. (B-C) Analysis of SOS closure in Tbx2b-depleted embryos. DIC images of eyes from live tbx2b+/+ (top panel) and tbx2bfby (bottom panel) embryos (B). Quantification of SOS closure in wild type and tbx2bfby mutant zebrafish eyes (C). Data are means ± SEM; one-way ANOVA with Tukey’s test: *P<0.05. Scale bars are 50 μm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
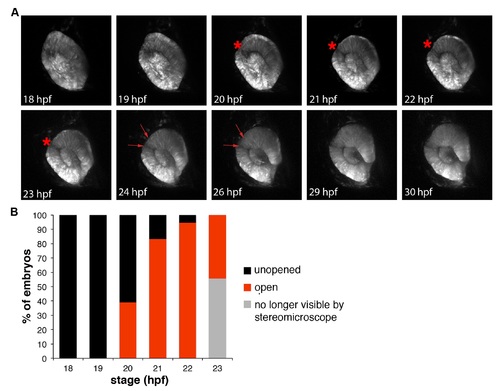
Dynamics of the zebrafish superior ocular sulcus. (A) Time-lapse images showing lateral views of the eye of a Tg(rx3:GFP) embryo. The superior ocular sulcus appears as a narrow groove across the dorsal retina at ~20 hpf (red asterisk), becomes wider by 24 hpf (red arrows) and disappears after 26 hpf. (B) Timing of SOS as viewed under a stereomicroscope. The wide and shallow phase is not visible by stereomicroscope, so the red bars indicate the percentage of embryos with a narrow and distinct sulcus. EXPRESSION / LABELING:
|
|
Reduced RA signaling does not impair closure of the superior ocular sulcus. (A) Lateral view of a 28 hpf zebrafish eye following in situ hybridization for cyp1b1. Note that expression extends into the dorsal eye. (B) Quantification of open SOS in 28 hpf embryos from cyp1b1+/- incrosses treated from 10 hpf with control solution or the Aldh inhibitor DEAB. N = 3 experiments, n = number of embryos. Data are means ± SEM. Statistics is two-way ANOVA with Tukey's test. Scale bar is 50 μm. ns, not significant. |
|
Human variant in BMPR1A reduces protein function. (A) One cell-stage zebrafish embryos were injected with caBMPR1A or R471H-caBMPR1A mRNA, and assessed at 24 hpf for morphological abnormalities by categorization according to the pictures shown. (B) Graph showing percentage of embryos injected with caBMPR1A (n = 22 embryos) or R471H-caBMPR1A (n = 23 embryos) that fit into each category of morphological abnormality. (C) qPCR showing equal amounts of injected RNA for each condition. Statistics is two-tailed t test. |
|
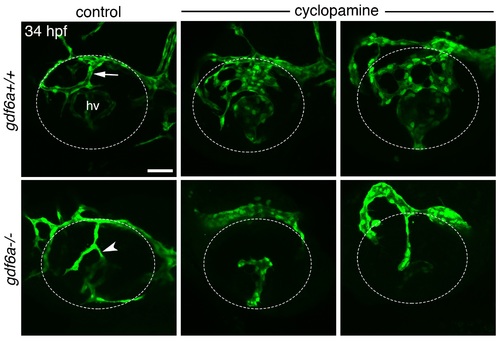
Interaction between Hedgehog and Bone Morphogenetic Protein signaling in formation of the dorsal radial vessel. Maximum projection confocal images of 34 hpf eyes from Tg(kdrl:eGFP);gdf6a+/+ and Tg(kdrl:eGFP);gdf6a-/- zebrafish embryos following treatment with control solution or 10 μM cyclopamine from 10 hpf. Blood vessels fluoresce green and the eye is outlined by dotted lines. DRV is indicated by arrow. Ectopic connection between superficial and hyaloid vasculatures indicated by arrowhead. Top row, right two panels are two examples of vessel overgrowth phenotype in cyclopamine-treated wildtype embryos. Bottom row, middle and right panels show the eyes of cyclopamine-treated gdf6a-/- embryos that either failed to form a DRV (n = 3/6 embryos) or grew a simple DRV that did not make an ectopic connection to the hyaloid vasculature (n = 3/6 embryos), respectively. hv, hyaloid vasculature. Scale bar is 50 μm. |
|
Retinoic acid signaling and the superior ocular sulcus. (A) Lateral views of eyes from 28 hpf zebrafish embryos that are gdf6a+/+, gdf6a +/-, or gdf6a-/- and have been processed for in situ hybridization. The top two rows show expression of the retinoic acid-synthesis genes cyp1b1 and aldha1a2. The bottom row shows expression of GFP in transgenic zebrafish carrying a reporter for RA signaling [Tg(12xRARE:GFP)] and are also gdf6a+/+, gdf6a +/-, or gdf6a-/-. Note reduced RA signaling in the superior retina of gdf6a +/- and gdf6a-/- embryos. (B) Graph showing no effect of retinoic acid treatment on SOS closure. Embryos from gdf6a+/- incrosses were grown from 10 hpf in control media, 5 nM retinoic acid, or 10 nM retinoic acid, and assessed at 28 hpf for an open SOS. (C) Graph showing no effect of the cyp1b1 mutation on SOS closure in gdf6a heterozygotes. gdf6a+/-;cyp1b+/- fish were crossed to wildtype or cyp1b-/- fish and the percentage of embryos with an open SOS was assessed at 28 hpf. n = number of embryos, N = 2 (B) or 3 experiments (C). Data are means ± SEM. ns, not significant |