- Title
-
Generation of all-male-like sterile zebrafish by eliminating primordial germ cells at early development
- Authors
- Zhou, L., Feng, Y., Wang, F., Dong, X., Jiang, L., Liu, C., Zhao, Q., Li, K.
- Source
- Full text @ Sci. Rep.
|
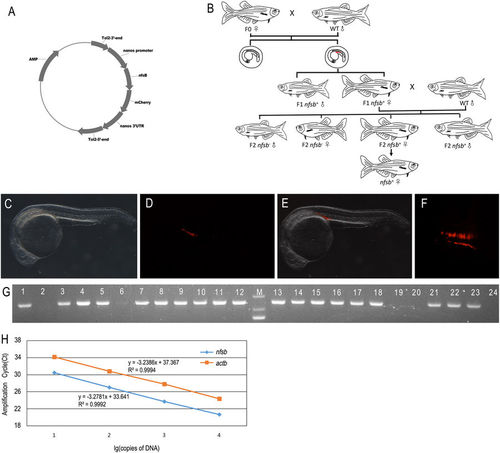
Generation of transgenic zebrafish of Tg(nanos3:nfsB-mCherry-nanos3 3′UTR). (A) Schematic diagram showing the construct used to generate the transgenic zebrafish line of Tg(nanos3:nfsB-mCherry-nanos3 3′UTR). (B) Schematic diagram showing the work flow of setting up the transgenic zebrafish. (C) Genotyping results of F1 zebrafish by PCR amplification. The gel picture was cropped from an original full-length gel image provided in Supplementary information. The identities of PCR products were further confirmed by Sanger sequencing. (D–F) Photomicrographs of the typical F2 embryos at 24 hpf displaying the expression of mCherry at the genital ridge (D: light; E: fluorescence; F: merge). Embryos were observed laterally. (G) PCGs with fluorescence were arranged as two lines. Embryos were observed dorsally. (H) Standard curve comparison. Standard curves for transgene Tg(nano3:nsfb-mCherry nanos3 3′UTR) and the endogenous β-actin gene (actb) in serially diluted (10-fold) DNA samples from the cloning vector of Tg(nano3:nsfb-mCherry nanos3 3′UTR) and wild type zebrafish genome. A very efficient amplification was obtained, as indicated by the slopes of the standards curves. Ct, cycle threshold. |
|
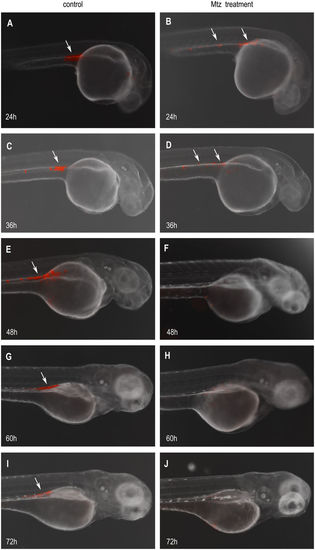
MTZ treatment eliminating the PGCs in the transgenic embryos. The PGCs (white arrow) marked with mCherry fluorescent were around anterior part of the yolk extension in Tg(nanos3:nfsB-mCherry-nanos3 3′UTR) transgenic embryos at 24 hpf (A), and were almost arranged in the genital ridge at 36, 48, 60, and 72 hpf (C,E,G,I), respectively. After treated with MTZ, the 24 hpf embryos exhibited the decreased number of PGCs with the weaker fluorescence (B). The number of PGCs was fewer and the fluorescent signal became weaker in the MTZ-treated embryos at 36 hpf (D). At 48 hpf, the fluorescence labeled-PGCs almost disappeared (F). No fluorescence labeled-PGCs were observed in the MTZ-treated embryos at neither 60 hpf (H) nor 72 hpf (J). |
|
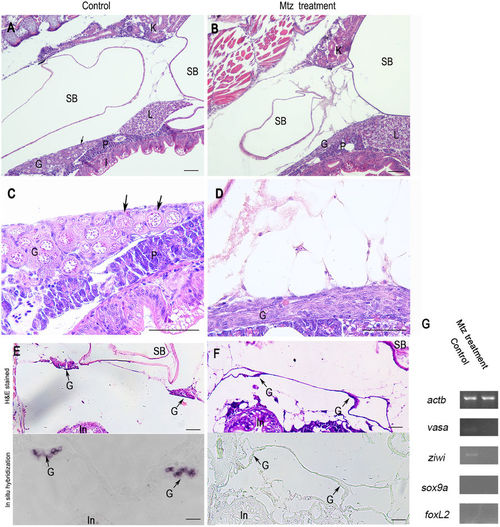
Germ cells ablation in 20-dpf zebrafish developed from MTZ-treated embryos. Longitudinal sections showing that the gonads of control zebrafish at 20-dpf (A and C) carried a large number of early perinucleolar oocytes (black arrow) with associated somatic tissue, whereas MTZ-treated zebrafish at 20-dpf had only somatic gonadal cells (B and D). Transverse sections showing that the gonads of control zebrafish at 20-dpf (E, upper panel) carried normal gonads (black arrow) with associated somatic tissue and had noraml vasa expression in gonads (E, lower panel, black arrow) detected by in situ hybridization, whereas the gonads of MTZ-treated zebrafish at 20-dpf had only somatic gonadal cells (F, upper panel) and had no vasa expression (F, lower panel) detected by in situ hybridization. (G) RT-PCR results showing the weak expressions of vasa and ziwi were detected in the gonads of control zebrafish but not in the zebrafish developed from the MTZ-treated embryos, and no expressions of sox9a and foxL2 was detetced in neither control zebrafish nor MTZ-treated zebrafish. Expression of actb was used as positive control. K: kidney, SB: swim bladder, G: gonad, L: liver, P: pancreas, I: Intestine. Scale bars = 20 µm (A,B,C,D) and 40 µm (E,F). |
|
MTZ-treated embryos develop into male-like adult zebrafish with shrunken testis 100-dpf adult zebrafish developed from control or MTZ-treated embryos. Both male and female zebrafish were found in the control (A), while all the adult zebrafish derived from the embryos treated with MTZ (B) were male-like with typical sexually pigmentation. The female/male ratio in control (C) or MTZ treatment group (D) were also analyzed. Dissected the testis from adult control and MTZ-treated zebrafish revealed that the testis of treated fish were shrunken, significantly smaller than those of controls (E,F). |
|
Histological analysis revealed the absence of germ cells at various developmental phases in the testis of MTZ-treated adult zebrafish. Transverse sections showing that the gonads of control zebrafish (A,C,D,G,H) and the ones of MTZ-treated zebrafish (B,E,F,I,J) at 100-dpf. At top of bilateral testis in the control adult zebrafish, germ cells with various developmental phases were found: spermatogonia, primary spermatocytes ScI, secondary speratocytes ScII, spermatids and spermatozoa (A), whereas MTZ-treated zebrafishes were found to have only Sertoli cells which were derived from somatic cells (B). Testis (arrow) near the bilateral gonadal junction also carried numerous seminiferous tubules with irregular shape in control adult fish (C,D), but there was no germ cells in the gonad (arrow) of the MTZ-treated fish at any developmental phases observed (E,F, arrow). Seminal vesicle-like structures were found in cross-section of the testis adjacent to the gonopore. The organ (arrow) consisted of numerous cavities communicated with each other, that contained a large quantity of sperm cells in the control zebrafish (G,H). No sperm was detected within the seminal vesicle in gonads (arrow) of the MTZ-treated zebrafish, so the cells of cavities which derived from somatic cells formed net structures (I,J). Transverse sections showing that the gonads of control male zebrafish at 100-dpf (K, upper panel) carried normal testis (black arrow) with germ cells at different developmental phases and had weak vasa expression in gonads (K, lower panel, black arrow) detected by in situ hybridization, the gonads of control female zebrafish at 100-dpf (L, upper panel) carried normal ovary and had strong vasa expression in developing oocytes (L, lower panel, black arrow) detected by in situ hybridization, whereas the gonads of MTZ-treated zebrafish at 100-dpf had only somatic gonadal cells (M, upper panel) and had no vasa expression (F, lower panel) detected by in situ hybridization. (N) RT-PCR results showing the expressions of vasa and ziwi were detected in the gonads of control male and female zebrafish, but not in the zebrafish developed from the MTZ-treated embryos, and strong expressions of sox9a and foxl2 were detetced in control male zebrafish and control female zebrafish respectively, weak expression of sox9a was found in control female zebrafish and MTZ-treated zebrafish, and no expression of foxl2 was detetced in control male zebrafish and MTZ-treated zebrafish. Expression of actb was used as positive control. Sg: spermatogonia, Sc I: Primary spermatocytes, Sc II: Secondary spermatocytes, St: Spermatids, Sz: Spermatozoa, SC: Sertoli cell, In: Intestine, M: Muscle, K: Kidney, Po: Primary oocyte, Vo: Vitellogenic oocyte. Scale bars = 20 µm. |