- Title
-
Shp2-MAPK signalling drives proliferation during zebrafish embryo caudal fin-fold regeneration
- Authors
- Hale, A.J., den Hertog, J.
- Source
- Full text @ Mol. Cell. Biol.
|
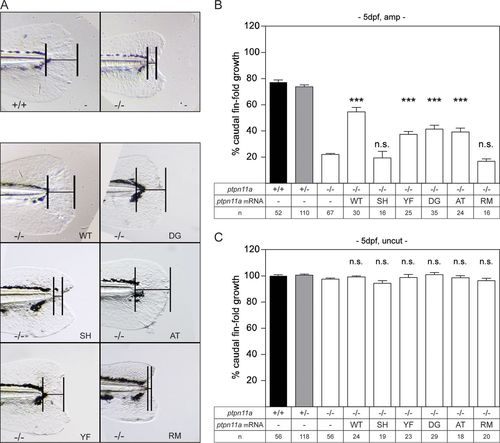
Functional Shp2a is required for regeneration. Zebrafish embryos from a ptpn11a+/− ptpn11b−/− incross were microinjected at the one-cell stage with synthetic mRNA encoding wild-type Shp2a, SH2 domain mutant Shp2a-R32M-R138M, C-terminal tyrosine mutant Shp2a-Y542F-Y580F, Shp2a-D61G, Shp2a-A462T, or Shp2a-R466M or were not injected (−). At 2 dpf, the caudal fin fold was amputated, and regeneration was assessed at 3 dpa (i.e., 5 dpf and 3 dpa); equivalent uncut controls were included (i.e., 5 dpf, uncut). All the embryos were genotyped. (A) Representative images of amputated ptpn11a−/− ptpn11b−/− embryo caudal fin folds at 3 dpa. A ptpn11a+/+ ptpn11b−/− sibling in which regeneration of the caudal fin fold was 80% complete by 3 dpa is shown for comparison (top left). (B and C) Regeneration was quantified by measuring the distance from the tip of the notochord to the edge of the caudal fin fold, as indicated (bars in panel A). The means of caudal fin fold growth are depicted relative to caudal fin fold growth of uncut ptpn11a+/+ ptpn11b−/− controls. Means of microinjected amputated (amp) (B) or uncut (C) ptpn11a−/− ptpn11b−/− embryos were compared to those of noninjected amputated or uncut ptpn11a−/− ptpn11b−/− embryos. The data were pooled from multiple experiments. Statistical evaluation was performed using a Mann-Whitney U test for comparison of ptpn11a−/− ptpn11b−/− zebrafish embryos with siblings within amputated or uncut groups, not between amputated and uncut groups. The error bars indicate standard errors of the mean. ***, P < 0.001; n.s. not significant. PHENOTYPE:
|
|
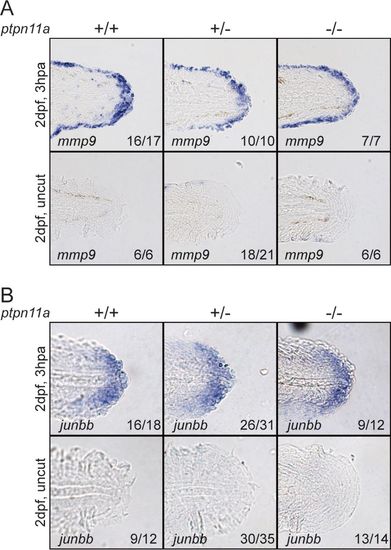
Formation of wound epidermis and distal blastema in zebrafish embryos lacking functional Shp2. At 2 dpf, the caudal fin folds of zebrafish embryos from a ptpn11a+/− ptpn11b−/− incross were amputated and allowed to regenerate. The embryos were fixed at 3 hpa, or the equivalent for uncut controls, and subjected to hybridization using probes specific for mmp9 (A) or junbb (B). Representative images of caudal fin folds of genotyped embryos are shown, and the number of embryos showing similar patterns/total number of embryos analyzed are indicated in the bottom right corner of each image. EXPRESSION / LABELING:
|
|
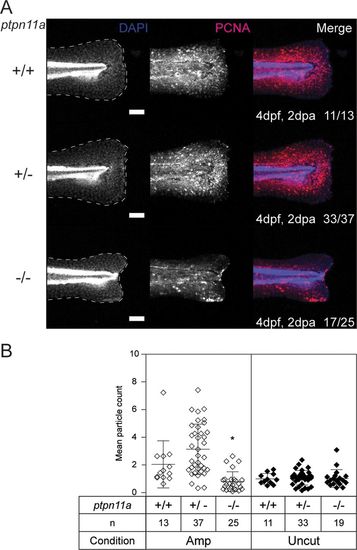
Proliferation is arrested at the amputated caudal fin fold margin of Shp2-deficient embryos. At 2 dpf, the caudal fin folds of embryos from a ptpn11a+/− ptpn11b−/− incross were amputated and allowed to regenerate. The embryos were fixed at 2 dpa (4 dpf, 2 dpa) and subjected to whole-mount immunohistochemistry using an antibody specific for the cell proliferation marker PCNA (red). The embryos were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (blue). Maximum-intensity projection images of the caudal fin folds were taken, and all the embryos were genotyped. (A) Representative images of amputated embryo caudal fin folds, with the edges of the fin folds indicated with dashed lines. The number of embryos showing similar patterns/total number of embryos analyzed are indicated in the bottom right corners of the images in the right-hand column. Scale bars, 100 μm. (B) PCNA immunofluorescence between the tip of the notochord and the edge of the caudal fin fold was quantified by mean particle count, with thresholding and size restriction to remove background signal. Equivalent uncut controls were also quantified, and the mean values of all the caudal fin folds are shown. The statistical significance of the means was determined relative to ptpn11a+/+ ptpn11b−/− zebrafish embryos within the amputated group, and likewise within the uncut group. *, P < 0.05; the error bars represent standard deviations. |
|
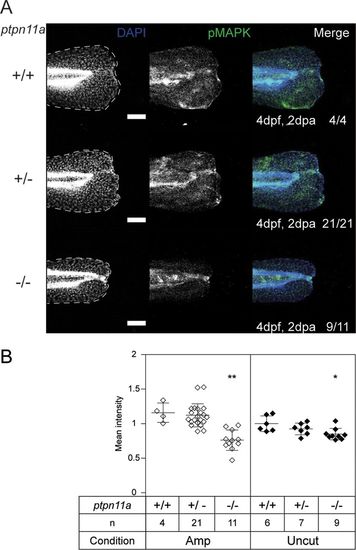
Reduced p-MAPK in regenerating caudal fin folds of Shp2-deficient embryos. At 2 dpf, the caudal fin folds of embryos from a ptpn11a+/− ptpn11b−/− incross were amputated and allowed to regenerate. The embryos were fixed at 2 dpa (4 dpf, 2 dpa) and subjected to whole-mount immunohistochemistry using a p-MAPK-specific antibody (Thr202/Tyr204) (green). The embryos were counterstained with DAPI (blue). Maximum-intensity projection images were taken of the caudal fin folds, and all the embryos were genotyped. (A) Representative images of amputated embryo caudal fin folds, with the edges of the fin folds indicated with dashed lines. The number of embryos showing similar patterns/total number of embryos analyzed are indicated in the right-hand column. Scale bars, 100 μm. (B) p-MAPK was quantified by the mean intensity of the region between the notochord and the edge of the caudal fin fold. Equivalent uncut controls were also quantified, and the mean values of all the caudal fin folds are depicted. The statistical significance of the means was determined relative to ptpn11a+/+ ptpn11b−/− zebrafish embryos within the amputated group, and likewise within the uncut group. **, P < 0.01; *, P < 0.05; the error bars represent standard deviations. |
|
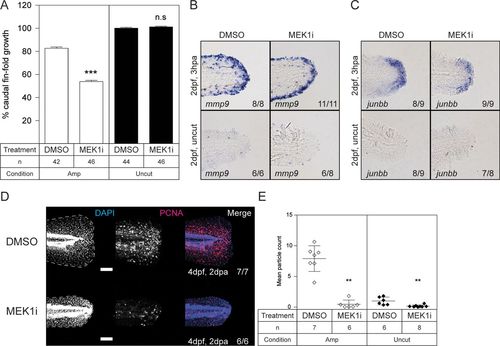
Impaired caudal fin fold regeneration in wild-type zebrafish embryos treated with MEK1 inhibitor. At 2 dpf, the caudal fin folds of wild-type embryos were amputated and allowed to regenerate in the presence of 50 nM PD184352 (MEK1i) or 1% DMSO (solvent control). (A) Regeneration after 3 days was quantified by measuring the distance from the tip of the notochord to the edge of the caudal fin fold. By 3 dpa, regeneration of the caudal fin fold of control zebrafish embryos was 80% complete. The means of caudal fin fold growth are depicted relative to caudal fin fold growth of DMSO-treated uncut controls. The statistical significance of the mean of PD184352 (MEK1i)-treated amputated embryos was determined relative to the mean of DMSO-treated amputated embryos, and likewise for the uncut treated and untreated embryos. The number of embryos is indicated (n). ***, P < 0.001; n.s. not significant; the error bars indicate standard errors of the mean. (B and C) Embryos were fixed at 3 hpa, or the equivalent for uncut controls, and subjected to hybridization for mmp9 (B) or junbb (C). Representative images of caudal fin folds of embryos are shown, and the number of embryos showing similar patterns/total number of embryos analyzed are indicated in the bottom right corner of each image. (D) Embryos were fixed at 2 dpa (4 dpf, 2 dpa) and subjected to whole-mount immunohistochemistry using an antibody specific for the cell proliferation marker PCNA (red). The embryos were counterstained with DAPI (blue). Maximum-intensity projection images were taken of the caudal fin folds. Representative images of amputated embryo caudal fin folds are shown, with the edges of the fin folds indicated with dashed lines. The number of embryos showing similar patterns/total number of embryos analyzed are indicated in the right-hand column. Scale bars, 100 μm. (E) PCNA immunofluorescence between the tip of the notochord and edge of the caudal fin fold was quantified by mean particle count, with thresholding and size restriction to remove background signal. Equivalent uncut controls were also quantified. The means of the amputated PD184352 (MEK1i)-treated group were compared to those of the amputated DMSO-treated group, and likewise, the means of the uncut PD184352 (MEK1i)-treated group were compared to those of the uncut DMSO-treated group. **, P < 0.01; the error bars represent standard deviations. |