- Title
-
Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity
- Authors
- Xu, B., Zhang, Y., Du, X.F., Li, J., Zi, H.X., Bu, J.W., Yan, Y., Han, H., Du, J.L.
- Source
- Full text @ Cell Res.
|
MiR-132 knockdown and mutation impair brain vascular integrity of larval zebrafish. (A, B) Representative images showing that miR-132 knockdown with morpholino oligonucleotides (MOs) caused intracranial hemorrhage (arrowheads) in wildtype larvae (A) and blood cell leakage (arrowheads) in the brain of Tg(Flk1:eGFP;Gata1:DsRed) larvae (B). The images were taken from larvae at 3 days post fertilization (dpf). (C) Summary of the intracranial hemorrhage effect of miR-132 knockdown. The experiments were repeated 4-8 times, and at each time > 89 embryos were examined for each group. (D, E) Representative images and summary showing that miR-132 knockdown caused more DAPI-positive brain parenchymal nuclei (blue) in 3-dpf Tg(Flk1:eGFP) larvae, in which a mixture of DAPI and Alex-568 dextran were injected into the blood circulation system. In D, the area outlined by the rectangles in the top are enlarged in the bottom, and the number of DAPI-positive nuclei in the area outlined by the dashed lines were counted. 13 control embryos and 18 morphants were analyzed in E. (F) Spatial distribution of leakage sites (red dots) in all 9 miR-132 morphants examined by in vivo time-lapse confocal imaging. (G-I) Transmission electron microscopy (TEM) images showing the ultrastructural changes of brain vessels in miR-132 morphants. The areas outlined by the red dashed rectangles in G were enlarged in (H, “Ctrl MO”) and (I, “miR-132 MO”). The green arrowheads in G point to the abnormal sites of the vessel luminal side, and the red arrowheads in I point to the abnormal junctions between vascular endothelial cells (ECs). (J) Rescue effect of miR-132RNA overexpression on intracranial hemorrhage defects in miR-132 morphants. The experiments were repeated four times, and at each time > 75 embryos were examined for each group. (K) Summary of the intracranial hemorrhage effect of miR-132 mutations generated by co-injection of zebrafish codon-optimized Cas9 mRNA and miR-132 gRNA. The experiments were repeated 3-5 times, and for each time > 21 embryos were examined for each group. Scale bar, 400 μm (top) and 100 μm (bottom) (A), 100 μm (B), 100 μm (top) and 10 μm (bottom) (D), 100 μm (F), 1 μm (G), 200 nm (H and I). Error bars, SEM. n.s., no significant; ***P < 0.001 (one-way ANOVA with post hoc Bonferroni's multiple comparison test for C and K; unpaired two-tailed Student's t-test for E; one-way ANOVA with post hoc Tukey's multiple comparison test for J). PHENOTYPE:
|
|
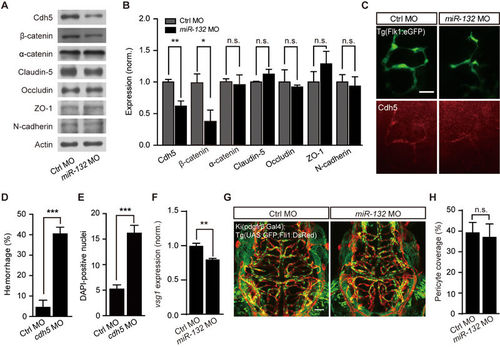
Involvement of Cdh5 in miR-132 knockdown-induced defects of brain vascular integrity. (A-C) Effects of miR-132 knockdown on the expression of junction proteins. Representative blots (A) and summary (B) of Western blot analyses. The experiments were repeated 3-8 times. (C) Cdh5 immunostaining in 3-dpf Tg(Flk1:eGFP) larvae. (D and E) Effects of Cdh5 knockdown on intracranial hemorrhage (D) and DAPI leakage (E) in 3-dpf larvae. The experiments were repeated 6 times and at each time > 49 embryos were examined for each group in D. 23 control embryos and 24 morphants were analyzed in E. (F) Effect of miR-132 knockdown on vsg1 expression. The experiments were repeated three times. (G, H) Representative projected confocal images (G) and summary (H) showing no significant effect of miR-132 knockdown on the pericyte coverage of brain blood vessels. 3-dpf larvae generated by crossing Ki(pdgfrβ:Gal4) with Tg(UAS:GFP;Fli1:DsRed) were used, and GFP and DsRed are expressed in pericytes and ECs, respectively. 6 control embryos and 6 miR-132 morphants were analyzed. Scale bar, 25 μm (C), 50 μm (G). Error bars, SEM. n.s., not significant; *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired two-tailed Student's t-test for B, D, E, F and H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Neuronal miR-132 regulates brain vascular integrity by affecting endothelial miR-132 level. (A) Relative expression level of miR-132 in zebrafish neurons and ECs, which were sorted by flow cytometry from 3-dpf Tg(HuC:GFP) or Tg(Flk1:eGFP) larvae, respectively. The experiments were repeated three times. (B-F) Effects of miR-132 sponge (miR-132-S)-mediated neuron-specific miR-132 downregulation on intracranial hemorrhage (B, C), DAPI leakage (D, E), and Cdh5 expression (F) in zebrafish larvae. The sponge was inserted into the tdTomato (tdT) 3′ UTR and driven by the HuC promoter (“HuC:tdT-miR-132-S”). Expression of tdT in neurons (“HuC:tdT”) served as a control. The experiments were repeated 10 times in C and 5 times in F, and 11 embryos injected with HuC:tdT and 8 embryos injected with HuC:tdT-miR-132-S were analyzed in E. (G) Coordinated changes of miR-132 level in zebrafish neurons and ECs, both of which were sorted from 3-dpf Tg(Flk1:eGFP) larvae transiently expressing HuC:tdT, HuC:tdT-miR-132-S or HuC:tdT-miR-132. Expression of HuC:tdT-miR-132-S or HuC:tdT-miR-132 was used to down- or upregulate miR-132 level in zebrafish neurons, respectively. The experiments were repeated 3-4 times. (H,I) Effects of miR-132-S expression in ECs on intracranial hemorrhage (H) and Cdh5 expression (I) in zebrafish larvae. The experiments were repeated nine times in H and six times in I. For each time, > 14 embryos were examined for each group in H. Scale bar, 400 μm (top) and 100 μm (bottom) (B), 100 μm (top) and 20 μm (bottom) (D). Error bars, SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired two-tailed Student's t-test for A, C, E, F, H and I; one-way ANOVA with post hoc Bonferroni's multiple comparison test for G). |
|
Neuronal exosomes are translocated to brain ECs in larval zebrafish. (A) In vivo time-lapse confocal images showing that a GFP-positive neuronal exosome (white arrowheads) approaches and enters RFP-positive ECs in the brain. The images were taken from a 3-dpf Tg(Flk1:RFP) zebrafish larva, in which HuC:CD63-GFP was transiently expressed to label neuronal exosomes. To trace the moving GFP-positive exosome at each time point, single-slice images at different optical sections are shown at the Left. To confirm the exosome internalization into ECs, three-dimensional views of the single-slice image at 90′ are shown at the Right. (B, C) Intracranial hemorrhage effects of nSMase2 blockade with spiroepoxide (B) or nSMase2 knockdown with MO (C) in zebrafish. The nSMase2 is required for the budding of exosomes into multi-vesicular bodies. The experiments were repeated seven times in B and six times in C. Scale bar, 5 μm (A). Error bars, SEM. ***P < 0.001 (unpaired two-tailed Student's t-test for B and C). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|