- Title
-
Aipl1 is required for cone photoreceptor function and survival through the stability of Pde6c and Gc3 in zebrafish
- Authors
- Iribarne, M., Nishiwaki, Y., Nakamura, S., Araragi, M., Oguri, E., Masai, I.
- Source
- Full text @ Sci. Rep.
|
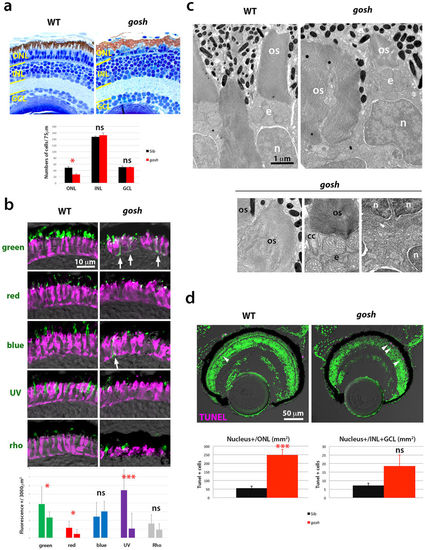
Photoreceptor phenotypes of the gosh mutant at 7 dpf. (a) (Upper) Sections of 7 dpf wild-type and gosh mutant retinas. In the gosh mutant, the ONL is thinner with abnormally shaped OS. However, INL and GCL appear to be normal. (Lower) Histogram of cell number of the ONL, INL, and GCL at 7 dpf in wild type (black) and gosh mutant (red). Numbers of nuclei within 75 μm length for each layer were counted (n = 3). Only ONL cells are significantly reduced in the gosh mutant (p = 0.026, students’ t-test). (b) (Upper) Labeling of wild-type and gosh mutant retinas with antibodies against green, red, blue, UV opsins, and rhodopsin (green), and zpr1 antibody (magenta). In the gosh mutant, the opsin-localized OS area is small, and opsins are often mislocalized to plasma membranes of cell bodies or synaptic areas (arrows). In contrast, rhodopsin localization to the OS seems to be normal. (Lower) Histogram of visual pigment-positive area at 7 dpf in wild type (left bars) and gosh mutant (right bars). The percentage of visual pigment-positive areas relative to a 3000 μm2 area containing the ONL was measured (n = 3 for each). Green, red, and UV opsin-positive areas were reduced in the gosh mutant, while there was no difference in blue and rhodopsin-expressing areas between gosh mutants and wild types. (c) EM images of wild-type and gosh mutant photoreceptors. The OS is composed of multiple, stacked photoreceptive membrane discs. Beneath the OS, mitochondria accumulate to form the ellipsoid (e). Although global shapes of the OS and nucleus (n) are deformed in gosh mutants, their fine structures, including the connecting cilium (cc), seem to be normal. Synaptic structures in the OPL appear to be less developed in the gosh mutant. (d) TUNEL of wild-type and gosh mutant retinas at 7 dpf (magenta). Apoptotic cells are indicated by arrowheads. Histogram of apoptotic cell number in the ONL (left) and the INL+GCL (right). In the gosh mutant, apoptotic cell number is markedly increased in the ONL. OS, outer segment; INL, inner nuclear layer; GCL, ganglion cell layer; n, nucleus; cc, connecting cilium; e, ellipsoid. (ns, p>0.05; *p<0.05; ***p<0.001). |
|
Cones are progressively eliminated in gosh mutants at 12 wpf. (a) (Upper) Sections of 12 wpf wild-type and gosh mutant retinas. In wild-type central retinas, rods and cones are distinguished. In gosh mutant central retinas, most ONL nuclei have a rod-like shapes, except for a single row of short single cones (red arrowheads), and an enlarged OS area. (a’) In the gosh mutant CMZ, both rods and cones are present. (Lower) Cell number of the ONL, INL, and GCL at 12 wpf in wild type (black) and the gosh mutant (red). Numbers of nuclei within 100 μm length for each layer were counted (n = 3). The ONL, INL, and GCL cells were reduced in the central retina of the gosh mutant. In the peripheral retina, ONL and INL cells was increased, but GCL cells were decreased in the gosh mutant. Students’ t-test: *p < 0.05; **p < 0.01. (b) Labeling of 12 wpf wild-type and gosh mutant retinas with anti-green opsin and rhodopsin antibodies (green), and zpr1 antibody (magenta). In wild-type central retinas, green opsin and rhodopsin are located in cone and rod OSs, respectively. However, in the gosh mutant, green opsin is absent, while most of the OS is labeled with rhodopsin. (b’) In the gosh mutant CMZ, both green opsin and rhodopsin are detected, although green opsin is mislocalized in some cells (arrows). A dotted line indicates the interface between the ONL and the OPL. (c) Twelve wpf wild-type ONL, which consists of small, compact rod nuclei, short and long single cones, and double cones (left), rod OS (right upper), and synapses (arrows show ribbon, right bottom). An arrowhead indicates the outer limiting membrane. (d) Twelve wpf gosh mutant ONL, which consists of rod nuclei and single row of cone-like cells (arrows, left), rod OS (right upper), and synapses (a white arrow shows the synaptic ribbon, right bottom). The outer limiting membrane is maintained at the outer border of rod nuclei (arrowhead). OS, the outer segment; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; olm, outer limiting membrane; ros, rod outer segment; cos, cone outer segment; e, ellipsoid; cc, connecting cilium. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The gosh mutant gene encodes aipl1b. (a) Schematic representation of human AIPL1 and zebrafish Aipl1b. FKBP and TPR domains are conserved. However, the zebrafish C-terminal region is not homologous to the PRD domain. In the gosh mutant, a nonsense mutation occurs in Gln413. (b) Phylogenetic tree of AIPL1 proteins of humans (Genbank no: AAF74023.1), mice (Genbank no: AAK77956.1), chicks (Genbank no: XP_001233085.3), Xenopus (Genbank no: XP_002933555.1), and zebrafish Aipl1a and Aipl1b. (c) Expression of aipl1a and aipl1b mRNAs in wild type at 72 hpf. aipl1b mRNA is expressed in the retina and the pineal eye (arrow). aipl1a mRNA is expressed in the brain, including the retina. Strong expression of aipl1a mRNA in the ventral patch of the retina. (d) Expression of aipl1a and aipl1b mRNAs in wild-type adult retina. aipl1b mRNA is expressed exclusively in cones, while aipl1a mRNA is expressed in rods and probably in short single (UV) cones (red arrowheads). ss, short single cone (red arrowheads); ls, long single cones (red outlined arrowhead); d, double cones (gray outlined arrowhead). (e) Expression of aipl1a and aipl1b mRNAs in wild-type and gosh mutant retinas at 72 hpf. aipl1b mRNA is markedly decreased in gosh mutant retinas, while aipl1a mRNA expression in ventral photoreceptors (arrows) is similar between wild-type and the gosh mutant retina. EXPRESSION / LABELING:
PHENOTYPE:
|
|
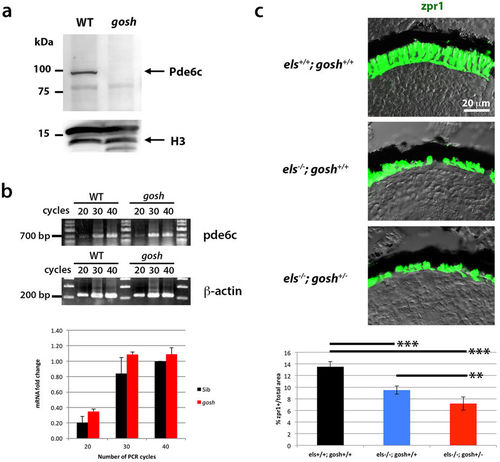
The gosh mutant genetically interacts with the els mutant. (a) Western blot of wild-type and gosh mutant heads with anti-Pde6c antibody. A band of approximately 100 kDa was detected in wild type animals, but disappeared in the gosh mutant. Histone H3 is as a loading control. (b) Semi-quantitative PCR with different numbers of cycles (20, 30 or 40) indicates that mRNA levels are similar between wild-type zebrafish and the gosh mutant. (c) Labeling of els+/+;gosh+/+, els−/−;gosh+/+, and els−/−;gosh+/− retinas with the zpr1 antibody. The ratio of zpr1-postive area relative to the total retinal area is significantly decreased in els−/−;gosh+/− retinas compared to els−/−;gosh+/+ retinas. ANOVA was significantly different (p< 0.000016) by t-test with a Bonferroni correction post-hoc (*p < 0.05; **p < 0.01; ***p < 0.001). |
|
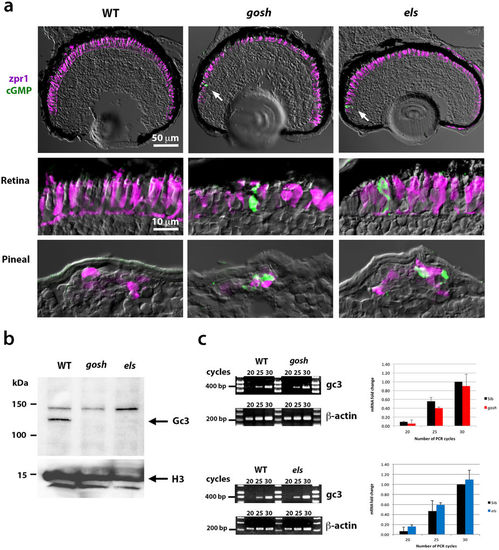
zGc3 expression is coupled to Aipl1 and Pde6c in zebrafish photoreceptors. (a) Labeling of wild-type, gosh, and els mutant retinas and pineal eyes at 7 dpf with anti-formaldehyde-fixed cGMP antibody (green) and zpr1 antibody (magenta). Control wild-type retinas show an undetectable level of cGMP. gosh and els mutant retinas show similar results, except that a few photoreceptors near the CMZ show very high accumulation of cGMP (arrow). On the other hand, pineal photoreceptors are positive for cGMP in gosh and els mutants, but not in wild type fish. (b) Western blot of wild-type, gosh and els mutant heads with anti-zGc3 antibody. A band of ~120 kDa is detected in wild-type heads, but disappears in both gosh and els mutant heads. (c) Semi-quantitative PCR of gc3 mRNA expression in wild-type, and gosh or els mutant embryos. mRNA levels are similar between wild-type, and gosh or els mutant embryos. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|
|
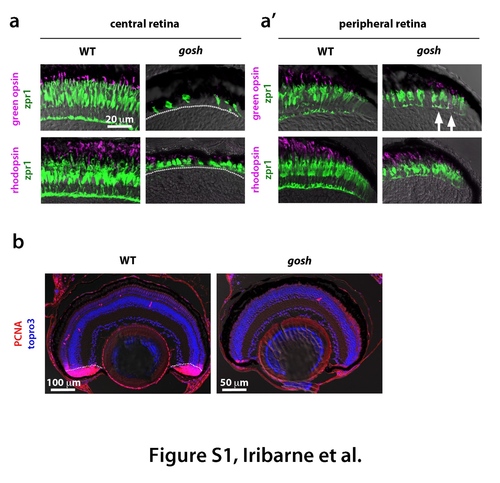
Degeneration of cone and rod photoreceptors at 4 wpf in the gosh mutant (a) Labeling of wild-type and gosh mutant retinas at 4 wpf with green opsin and zpr1 antibodies, or rhodopsin and zpr1 antibodies. In wild-type central retina, the ONL is thicker than at 7 dpf to segregate rod and cone nuclear layers. Its cone shape is highly elongated. Opsin and rhodopsin are localized in the OS. In gosh mutant central retina, the ONL is very thin, and cones are fewer in number and abnormal in shape. (a') In wild-type peripheral retina, new rod and cone photoreceptors are generated and express rhodopsin and opsin, respectively. In gosh mutant peripheral retina, cones become columnar although their thickness is still less than in wild type retina. Green opsin and rhodopsin are detected; however, green opsin is mislocalized through the cell body (arrows). A dotted line indicates the boundary between photoreceptor cells and INL. (b) Labeling of wild-type and gosh mutant retinas of 2 wpf with PCNA antibody (red). Nuclei are counter-stained with TOPRO3 (blue). In wild-type retina, most of the CMZ express PCNA, indicating cell proliferation of retinal stem and progenitor cells (dotted line). In wildtype retina, PCNA positive cells are observed just beneath the ONL and in the INL, which correspond to rod progenitors and Müller cell-derived regenerating progenitors, respectively. In contrast, PCNA expression is reduced in the gosh mutant CMZ, in the number of rod progenitors and Müller cell-derived regenerating progenitors, suggesting that retinal regeneration is not active in the gosh mutant at 2 wpf. |
|
Generation of a new allele of the gosh mutant using the CRISPR/Cas9 system (a) Sequence of exon1 of the aipl1b gene in embryos showing no OKR response. A 17-bp insertion causes a frame-shift of the coding region, which subsequently induces a premature stop codon. (b) Diagram showing the strategy used to confirm that gosh mutant gene encodes aipl1b. (c) Labeling of retinas of 7-dpf no-OKR zebrafish embryos with anti-blue opsin and zpr1 antibodies or anti-rhodopsin and zpr1 antibodies. Photoreceptors are abnormal in shape, and blue opsin is mislocalized to the plasma membrane of the cell body (arrows). However, rhodopsin is normally localized at the OS. |
|
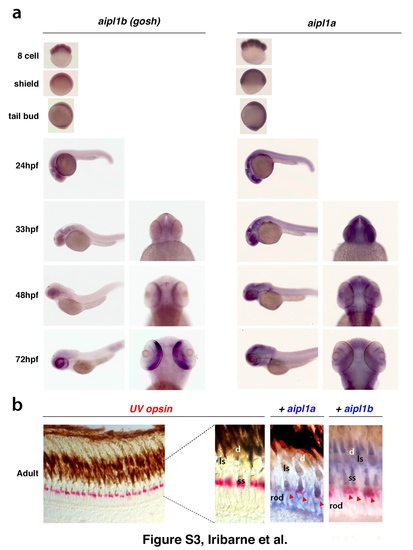
Expression of aipl1 mRNA during embryonic development (a) Whole-mount in situ hybridization of wild-type embryos from the 8-cell stage to 72 hpf using aipl1b and aipl1a RNA probes. (b) (Left panel) In situ hybridization of cryo-sectioned wild-type adult retina with UV opsin RNA probe (red). UV opsin is expressed in short single cones, which are regularly located just above the nuclear layer of rod photoreceptors. (Right panels) Wild-type adult retinas were co-labeled with the UV opsin RNA probe (red), and the aipl1a or aipl1b probe (blue). aipl1a andaipl1bmRNAs are expressed in rods and cones, respectively. Only short single cones are positive for both aipl1a and aipl1b probes (red arrowhead). Abbreviation: ss, single short cone; ls, long single cone; d, double cone. |
|
Labeling of wild-type, gosh, and els mutant retinas with anti-cGMP antibody Cryo-sectioned heads of 4-dpf wild-type, gosh, and els mutant embryos are labeled with antiformaldehyde- fixed cGMP antibody (green) and zpr1 antibody (magenta). As in 7-dpf samples (Fig. 5a), wild-type, gosh, and els mutant retinas show undetectable levels of cGMP, although a few photoreceptors with high levels of cGMP were occasionally observed in the CMZ of gosh and els mutant retinas (arrows in gosh mutant panel). High levels of cGMP were observed in pineal photoreceptors in both mutants (arrow in els mutant panel). |