- Title
-
A Zebrafish Model for Chlamydia Infection with the Obligate Intracellular Pathogen Waddlia chondrophila.
- Authors
- Fehr, A.G., Ruetten, M., Seth-Smith, H.M., Nufer, L., Voegtlin, A., Lehner, A., Greub, G., Crosier, P.S., Neuhauss, S.C., Vaughan, L.
- Source
- Full text @ Front Microbiol
|
Infection of zebrafish larvae with W. chondrophila via bath-immersion at 4 dpf followed by IF staining with an anti-Waddlia antibody (red) and DAPI (blue). Section view of a CLSM acquired 3D stack of a larva at 4 hpi. The section view exhibits a large amount of swallowed W. chondrophila inside the lumen of the intestine (A). Fluorescence light-microscope appearance of the trunk region of a larva at 48 hpi shows in addition to the accumulation of W. chondrophila in the gut lumen (arrow), an infection of the swim bladder with bacterial inclusions (arrow heads) visible inside the epithelium (B). |
|
Swim bladder infection in 4–5 dpf old larvae. Microinjection of W. chondrophila EBs directly into the lumen of the swim bladder of 4 dpf larvae. A drop of approx. 1 nl of the bacterial suspension can be seen hanging on the tip of the injection needle (arrow) inside the air filled lumen of the swim bladder (A). CLSM acquired 3D stack of the trunk region of a larva at 36 hpi, after IF staining with an anti-Waddlia antibody (red) and DAPI (blue). The swim bladder in the center of the image exhibits several bacterial inclusions (arrow heads) inside the epithelium (B). Histology on HE-stained sections of the swim bladder of a PBS injected control larva (C) with normal appearance and of an infected larva (D) showing clear pathological changes, including thickening of the epithelium (E) and infiltration of innate immune cells like macrophages and neutrophils (F). Scale bars (A–D) 100 μm, (E) 50 μm, and (F) 20 μm. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Light-microscope appearance of a transgenic Tg(fli1a:eGFP) larva at 48 hpi after intravenous injection of live bacteria (A), showing an impaired blood flow ending in an almost completely stopped circulation, accompanied by pericardial oedema formation (arrow). Section view of a CLSM acquired 3D stack (B) after IF staining with an anti-Waddlia antibody, showing the infection of GFP expressing endothelial cells (green) with W. chondrophila (red). Host cell nuclei were stained with DAPI (blue). Surface rendering of the tail artery and caudal vein shows the distribution of the inclusions inside the vasculature at 24 hpi (C) and 36 hpi (D), showing bacterial spread across endothelial bounds (arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
CLSM acquired 3D image of W. chondrophila inclusions inside the swim bladder epithelium (A) after IF staining of Waddlia (red), mitochondria (green) and DAPI staining of host cell and bacterial DNA (blue). The image shows the formation of several bacteria containing vacuoles inside epithelial cells, accompanied by the recruitment and close association with host cell mitochondria. Close-ups of single inclusions with CLSM (B) and TEM (C) reveal typical features of the chlamydial life cycle, such as transformation from the smaller infectious EB with condensed DNA (arrow) to the larger and metabolically active RB form with finely distributed chromatin (arrow head), dividing inside the vacuole. TEM images show also the morphology of infected epithelial cells of the swim bladder (D) and endothelial cells of blood vessels (E), usually containing a single perinuclear inclusion (asterisks), strongly associated with host cell mitochondria. Scale bars (A) 2 μm, (B,C,E) 1 μm and (D) 5 μm. |
|
Neutrophil recruitment and uptake of W. chondrophila monitored in transgenic Tg(lyzC:dsRednz50) larvae at 8 hpi. Whilst the swim bladder of a healthy larva is usually nearly devoid of innate immue cells (A), the injection of W. chondrophila into the swim bladder activates strong neutrophil recruitment (B). Quantification of recruited neutrophils (C) shows a significantly increased reaction to live W. chondrophila (live) compared to heat-inactivated (inact) bacteria or with sterile PBS injected control larvae, ***p < 0.001. After the uptake by a neutrophil W. chondrophila can successfully avoid its degradation and instead start replicating inside the phagosome to form an inclusion shown by 3D-CLSM (D) and TEM (E). (D) shows a 3D acquired z-stack image of a dsRed expressing neutrophil (red) of the transgenic Tg(lyzC:dsRednz50) line, harboring three W. chondrophila inclusions, visualized by antibody staining (green). DNA was stained with DAPI (blue). The TEM image in (E) shows a zebrafish phagocyte containing two inclusions of replicating W. chondrophila RBs. Scale bars (A,B) 100 μm, (D) 1 μm and (E) 2 μm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
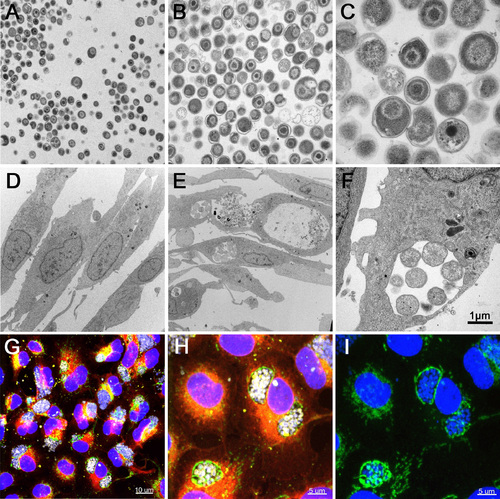
Infection of EPC cells with W. chondrophila. (A-C) TEM of W. chondrophila EBs, freshly harvested from an amoeba co-culture and injected into eggwhite. (D) TEM of non-infected EPC cells. (E) TEM of EPC cells, infected with W. chondrophila. (F) TEM of a W. chondrophila inclusion, containing replicating RBs and surrounded by host cell mitochondria. (G-I) IF staining of infected EPC cells with concanavalin A (red), an anti-Waddlia antibody (yellow), an anti-OxPhosIV antibody to stain mitochondria (green) and DAPI. The perinuclear BCVs show close association with host cell mitochondria. (I) DAPI and anti-OxPhosIV antibody alone. |