- Title
-
Collagen XII Contributes to Epicardial and Connective Tissues in the Zebrafish Heart during Ontogenesis and Regeneration
- Authors
- Marro, J., Pfefferli, C., de Preux Charles, A.S., Bise, T., Jaźwińska, A.
- Source
- Full text @ PLoS One
|
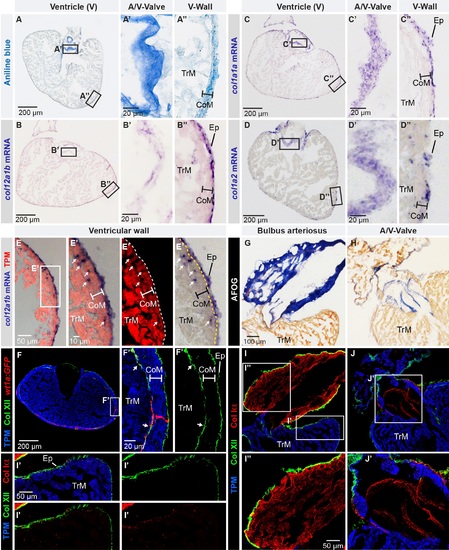
Col XII is expressed in the epicardium of the adult zebrafish heart. (A) Aniline blue staining of a ventricle transversal section visualizes collagen (blue). Framed areas encompass parts with the atrio-ventricular valve (A/V-Valve) and ventricular wall (V-Wall). The thickness of the compact myocardial layer is depicted as a bar in this and subsequent panels. Ep, epicardium; CoM, compact myocardium; TrM, trabecular myocardium. N = 5. (B-D) In -situ hybridization of ventricle sections detected by a color reaction (purple). Probe names are to the left. Framed areas encompass the parts that are enlarged in the panels to the right. N ≥ 4. (E) Superposition of a bright-field image with in -situ hybridization using col12a1b probe (purple) and fluorescent immunodetection of muscle protein Tropomyosin, TPM (red). col12a1b is expressed in the epicardium that is located externally from the myocardial border (dashed line). A few col12a1b-expressing cells are Tropomyosin-negative (arrows) and are interspersed within the compact myocardium (the thickness of the compact myocardium is indicated with a white bar). N = 4. (F) Immunofluorescence with anti-Tropomyosin (blue) to label cardiomyocytes and anti-Col XII (green) of transgenic fish wt1a(-6.8kb):GFP (red), which labels cardiac subepicardial fibroblasts (white arrows) located mainly along the junction between the outer compact myocardium (white bar) and inner trabecular myocardium. N = 4. (G, H) Aniline blue, acid Fuchsin, Orange G (AFOG) staining detects collagen (blue) in bulbus arteriosus (G, longitudinal heart section) and the leaflets of the atrioventricular valve (H, transversal heart section). N = 6. (I, J) Triple immunofluorescence staining against Col XII (green), Col Iα (red) and Tropomyosin (blue) of the structures shown in above panels. (I’) Col XII is detected on the myocardial surface. (I”) In the bulbus arteriosus, Col Iα fibers are in the interstitium, while Col XII is restricted to its surface. (J’) The atrio-ventricular connection displays Col Iα, but little Col XII in the valve leaflets. N = 6. (A’, B’, C’, D’, E’, F’) Higher magnifications of the framed areas shown in images that are labeled with the same letter without prime symbol. The same rule applies to all subsequent figures. |
|
Developmental dynamics of Col XII expression in the zebrafish heart. (A-D) Longtudinal sections of the zebrafish heart after double immunostaining against Col XII (green) and Tropomyosin (red), with DAPI contrastain (blue). dpf, days post-fertilization. Three chambers of the zebrafish heart: v, ventricle; a atrium; b.a., bulbus arteriosus (non-muscular structure). N ≥ 5. (A) At 3 dpf, embryos express Col XII in the pericardium, but not in the heart. The pericardial fibers seem to invade the surface of the heart. (B) At 14 dpf, the three chambers of the larval heart are surrounded by Col XII-positive fibrils within 10 μm of outer myocardial layer (white bar). (C) At 30 dpf, the juvenile fish heart contains a thickened myocardium (red), but the size of Col XII-positive layer remains unaltered. (D) At 120 days post fertilization, young adult fish maintain Col XII-labeled fibers along the heart circumference in a pattern similar to the one seen at the larval stage. |
|
Collagen XII is transiently upregulated during heart regeneration in adult zebrafish. (A-J) Immunostaining of transversal sections of ventricles at different time points after cryoinjury. The post-infarcted tissue is defined as a Tropomyosin-negative part of the ventricle (encircled with a dashed line). (A, B) At 4 dpci, the epicardial layer with Col XII becomes markedly thicker at the site of injury. N = 8. (C, D) At 7 dpci, the border zone of the remaining myocardium contains a few Col XII-positive fibrils (arrows). N = 8. (E, F) At 14 dpci, the post-cryoinjured tissue displays accumulation of Col XII-containing matrix. N = 7. (G, H) At 28 dpci, new cardiomyocytes replace the provisional tissue, leading to the progressive decrease of the post-cryoinjured matrix. Col XII persists in the leading edge of the regenerating myocardium. N = 5. (I, J) At 60 dpci, heart regeneration has been accomplished. The expression of Col XII is reminiscent of the original pattern of uninjured hearts. N = 8. (K) Immunofluorescence staining of ventricle sections using antibodies against Col XII (green) and embryonic cardiac myosin heavy chain (embCMHC, red). At 7 dpci, undifferentiated cardiomyocytes along the regenerating leading edge are associated with the Col XII-containing ECM. N = 6. (L) The percentage of Col XII-positive area within the fibrotic tissue of the post-cryoinjured myocardium without epicardial expression. N ≥ 5; **P < 0.01, **** P < 0.0001. |
|
Collagen XII distribution correlates with the activated epicardium and fibroblast-like cells. (A-F) Analysis of transversal heart sections at 14 dpci. (A and D) AFOG staining of the sections used for immunostaining. (B and C) Raldh2 expression (red) demarcates the activated epicardium and endocardium. (C') Col XII (green) and Raldh2 are colocalized in the intact epicardium (epicard). (C'') Raldh2-positive cells invade the post-cryoinjured area that is labeled by Col XII expression. Cardiac muscle is detected by Tropomyosin antibody staining (blue). N = 5. (E and F) Triple antibody staining against Col XII (green), intermediate filament Vimentin (blue) and alpha-Smooth Muscle Actin (αSMA; red). (F' and F'') αSMA- and Vimentin-positive cells are non-overlapping cell populations in the epicardium and post-cryoinjured area. Both of them are associated with Col XII-labeled fibrils. N = 6. |
|
The multi-component matrix of the fibrotic post-cryoinjury tissue is rich in Collagen XII. (A) Heart section of transgenic fish wt1a(-6.8kb):GFP (red) at 14 dpci. (A’) Subepicardial fibroblasts (red) co-localize with Col XII deposition (green). (A'') The injury zone contains wt1a(-6.8kb):GFP-positive cardiac fibroblasts that are associated with Col XII-positive matrix. N = 5. (B-G) Higher magnifications of fibrotic wound tissue of sections at 14 dpci that were triple immunostained and then used for histological coloration with AFOG. The intact myocardium is detected by Tropomyosin staining (blue); Col XII (green). (B, D, and F) AFOG staining of the corresponding sections used for immunostaining. (C) Col XII and Col Iα (red) are co-distributed in the post-cryoinjured area. (E) Col XII and Tenascin C (red) partially co-localize. (G) Fibronectin (red) partially overlaps with Col XII. N ≥ 4. |
|
TGF-β signaling is activated in Collagen XII-positive fibrotic tissue. (A-C) Analyses of ventricle sections at 14 dpci. (A) In -situ hybridization detects tgf-β2 expression in the cryoinjury area. N = 5. (B, C) Immunofluorescence staining reveals co-distribution of Col XII fibrils (green) and p-Smad3 (red) in the post-cryolesion zone that is recognized by the absence of Tropomyosin (blue in B). p-Smad3 reactivity is detected in the nuclei visualized by DAPI (blue in C). N = 5. |
|
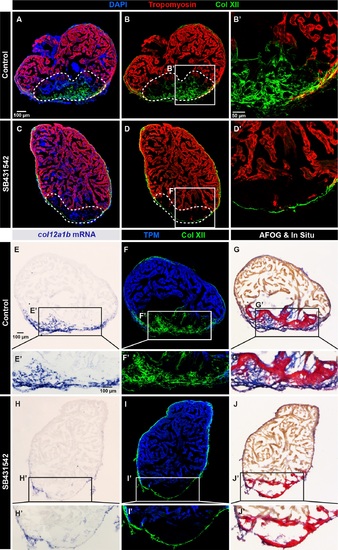
Collagen XII deposition in the post-cryolesion tissue requires TGF-β signaling. (A, B) Immunofluorescence staining shows a normal pattern of Col XII (green) in the Tropomyosin (red)-negative area in control heart. N = 5. (C, D) The treatment with the inhibitor of the TGF-β receptors, SB431542, suppresses the Col XII deposition (green) in the post-cryolesion area, without affecting the pre-existing epicardial fibrils. N = 7. (E-J) In -situ hybridization, double immunostaining and AFOG coloration of control (E-G) and SB431542-treated (H-J) hearts. (E, F) In -situ hybridization and fluorescent staining reveals that col12a1b transcripts (blue) match the distribution of Col XII protein. (F) Col XII fibrils (green) are present in the post-cryolesion area that is recognized by the absence of Tropomyosin (blue). (G) AFOG staining highlights the intact muscle (orange), fibrin-like matrix (red) and collagen (blue) that overlaps with the in -situ staining. (H) The inhibition of TGF-β signaling prevents upregulation of col12a1b expression in the post-cryoinjury area, without affecting its epicardial expression. (I) The cryoinjured zone is devoid of Col XII. (J) AFOG coloration reveals the absence of collagenous matrix (blue) in the post-infarcted zone. N = 6. |
|
The upregulation of fibrillar Collagen Iα in the post-cryoinjured area is induced by TGF-β signaling. (A-D) Immunofluorescence staining of heart at 14 dpci using Col Iα (green) and Tropomyosin (red). The post-infarcted tissue is tropomyosin-negative (encircled with a dashed line). (A-B') Control heart displays the presence of fibrillar collagen in the fibrotic tissue. N = 4.(C-D') The treatment with the inhibitor of the TGF-β receptors, SB431542, suppresses Col Iα (green) in the inner wound site. In the epicardium, Col Iα can be still detected (arrowhead). N = 6. (E-H') In-situ hybridization against col1a2 (purple) and immunostaining with Tropomyosin (red). (E-F') Control hearts display expression of col1a2 in the post-infarcted tissue. (G-H') The inhibition of TGF-β signaling with SB431542-treated suppresses col1a2 expression in the inner part of the wound, without affecting the epicardial expression. N = 6. |
|
The adult zebrafish heart and its collagenous components. (A-B) Aniline blue, acid Fuchs in and Orange G (AFOG) histological staining demarcates the muscle of the ventricle (orange) and non-muscular structmes that are rich in collagen (blue). (A) The longitudinal section of the zebrafish heart shows a pyramidal shape of the ventricle and peer-shaped bulbus arteriosus of the outflow tract. The atrium is not included in the section, but the atria-ventricular valve is visible. (B) Transversal section of the zebrafish ventricle at the level of the atrio-ventricular valve. Magnified images of the ventricular wall (framed areas) reveal an outer layer ofa compact myocardium, which surrounds the main spongy (trabecular) myocardium. Little collagen (blue) is visible in the epicardium. (C) In-situ hybridization of tJ1e heart ventricle display a weak expression of coll 2a I a in tJ1e uninjured zebra fish heart. (D) Predicted mature protein structures ofzebrafish FACIT collagens: Collagen XII alpha la (Col XIlala) and Collagen XII alpha la (Col XII a I b); and fibril-forming collagens: Collagen I alpha I a (Col Ia I a) and Collagen I alpha 2 (Col la2), based on the conserved domain analysis of the annotated genes in the public database. Pre-pro-peptide sequences are not shown. |
|
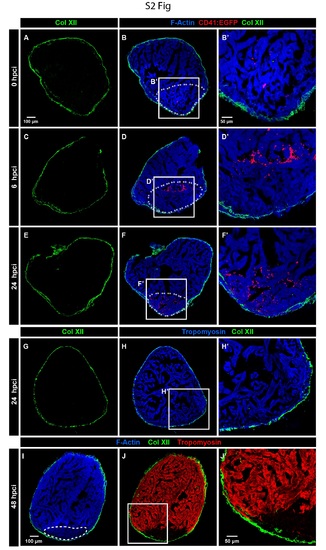
Croyinjury does not damage the pre-exisiting Col XII 1>rotein in the epicardium. (A-F') Immunofluorescence imaging oftJ1e cryoinjured hearts of transgenic fish Tg(CD4!:GFP) that visualizes tJ1rombocytes (false colored red). The site of injury is demarcated by U1e accumulation ofthrombocytes. The injured area does not display any change of muscle protein F-Actin (Phalloidin, blue) and epicardial Col XII (green). Dashed line surrounds the predicted injured zone based on U1e presence ofthrombocytes. Within 24 bpci, the expression ofColXII remains unaltered. (G-H') At 24 hpci, hearts displayed unaltered expression of the cytoskeletal protein Trompoyosin (blue) in the muscle and Col XII (green) in the epicardium. (I-J') At 48 hpci, the injured myocardium can be detected by the lower levels of F-Actin (detected by Phalloidin, blue) and complete absence ofTropomyosin (TPM, red). Col XII-positive fibrils in Ule epicardium remain unaltered around the cryinjured zone. |
|
col12a1a-expressing cells accumulate at the site of injury at the onset of heart regeneration. (A-K) In-situ hybridization of heart sections at early time points after cryoinjury with probes of two col12 paralogs (purple) followed by immunofluorescence staining against Tropomyosin (blue) and Col XII (green). (A-D) Uninjured hearts do not markedly express col12a1a, but col12a1b in the epicardium, which is surrounded by the Col XII deposition. (E-G) At 1 dpci, col12a1a-expressing cells cover the circumference of the injured heart, Col XII persists at the epicardium at the side of injury. (I-L) At 4 dpci, col12a1a- and col12a1b-expressing cells accumulate at the surface of the injury site. The gene expression correlates with the localization of Col XII protein. |
|
Complete ventricle sections that were used for analysis of fibrotic tissue at 14 dpci in Figure 5. (A-I) The framed areas correspond to the selected regions that are shown at higher magnification in Figure 5. Dashed line encircles the fibrotic tissue. (J-L') Chondroitin sulfate (red) does not markedly co-localize with Col XII (green). |
|
Treatment with 20 µm SB431542 reduces the activity of the TGFβ signaling pathway in injured hearts. (A, B) Immunofluorescence imaging of the control cryoinjured hearts detects the presence of pSmad3-positive cells within the peri-injured mycocardium (Tropomyosin-positive area, red) and fibrotic tissue (encircled by a dashed line). Exposure to the inhibitor of TGFβ signaling (SB431542) markedly reduces the number of pSmad3 immunoreactivity. (C) Quantification of the pSmad3-positive cells shown as the percentage of DAPI-labeled nuclei. N = 6. ** P = 0.002. |