- Title
-
Embryonic origin and lineage hierarchies of the neural progenitor subtypes building the zebrafish adult midbrain
- Authors
- Galant, S., Furlan, G., Coolen, M., Dirian, L., Foucher, I., Bally-Cuif, L.
- Source
- Full text @ Dev. Biol.
|
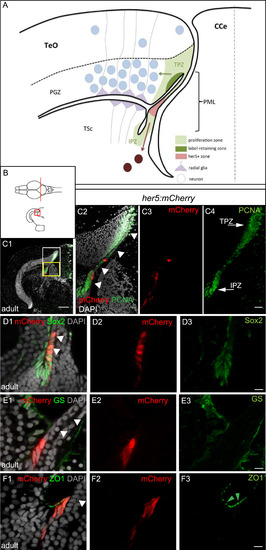
Adult her5-positive cells of the PML express the characteristics of neuroepithelial progenitors. A, B: Schematic cross-section of one tectal hemisphere from an adult zebrafish (level indicated in B, dorsal up, midline indicated by the vertical broken line) showing the different relevant cell populations (color-coded, see keys on figure). Arrows indicate the known lineages, ie: green arrow: generation of PGZ neurons and radial glia from TPZ proliferating progenitors ( Alunni et al., 2010 and Ito et al., 2010); dark red arrow: generation of tegmental neurons by her5-positive cells of the PML ( Chapouton et al., 2006). C: Cross section of an adult optic tectum at the level indicated in B, immunostained for mCherry and PCNA (color-coded) and counterstained with DAPI, position of the PML (arrowheads) and the Her5-mCherry-positive domain compared to the TPZ and IPZ. C1: low magnification showing one hemisphere, C2-C4: high magnifications of the white boxed area in C1 (C2: red and green channels with DAPI, C3: red channel only, C4: green channel only). Arrowheads to the PML on panel C2. D-F: compared expression of Her5-mCherry with markers for progenitors (Sox2), glial cells (GS) and tight junctions in neuroepithelial cells (ZO1, green arrowheads) on high magnifications of the PML area on cross sections of an adult optic tectum (C1-F3: High magnification of the yellow boxed area in C1, panels 1: red and green channels with DAPI, panels 2: red mCherry channel only, panels 3: green channel only). White arrowheads to the PML on panels 1. Scale bars: C 50 µm, D-F 10 µm. Abbreviations: CCe: crista cerebellaris, IPZ: isthmic proliferation zone, PGZ: periventricular grey zone, PML: peripheral midbrain layer/posterior midbrain lamina, TeO: tectum opticum, TPZ: tectal proliferation zone, TSc: torus semi-circularis. |
|
The her5-positive, TPZ, her4-positive and mature radial glia domains are spatially ordered in the posterior TeO. All views are confocal images. A, B. Compared expression of Her4-RFP, GFAP-GFP and GS (A) or PCNA (B) on cross-sections of the adult midbrain in double transgenic animals (high magnifications of the area boxed in the schematic). Panels 1 are RFP, GFP and GS merge views with DAPI counterstaining, Panels 2–4 are single channel views (color-coded). Note that Her4-RFP-positive glial cells (red arrows) extend into a more lateral ventricular domain than mature RG (A 1–4, B3, green or blue arrows point to the lateral limits of GFAP-GFP and GS expression, respectively) and reach into the PCNA-positive domain (B4, white arrow). C-F. Compared position of the Her5-mCherry (red, red arrows) and Her4-GFP (green, green arrows) positive territories in the posterior midbrain at increasing stages from embryo to adult, observed on cross sections (levels and stages indicated on the schematics). C′-F′. Interpretative drawings of C-F highlighting in red the her5-positive zone and in green the area covered by the cell bodies of her4-positive RG. Scale bars: A,B 100 µm, C-F 50 µm. Abbreviations: Cb: cerebellum, TeO: tectum opticum, TPZ: tectal proliferation zone, TSc: torus semi-circularis, V: ventricle. |
|
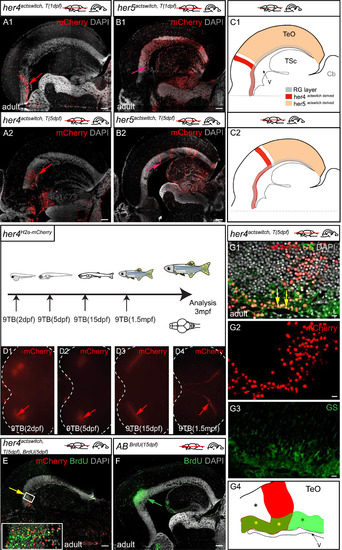
Post-embryonic her5-positive cells contribute to the generation of the posterior TeO via a TPZ intermediate. A-D. Adult fate of her5-positive cells assessed using Cre-lox recombination at the indicated stages (top schemes) and analysed in whole-mount brains (panels 1, dorsal views, anterior left, dotted line to surround the TeO) or on horizontal sections (panels 2 and 3, confocal views, with high magnifications of boxed areas in panels 3). The anterior limit of the TeO domain issued from recombined cells is indicated by yellow arrowheads in panels 1 and 2. Small red arrow in panel D2 point to transversal mCherry-positive polyclones (see high magnification in E). White arrowheads in panels 3 point to the PML, containing mCherry-positive cells (red arrowhead in D3) (in average, 15% ±4% cells per PML, n=2 fish and 6 sections analysed). E, F. mCherry-positive polyclones induced by a mosaic recombination in her5actswitch fish at 5dpf and analysed at adult stage. Confocal views of triple-immunostained horizontal sections (areas boxed in scheme), merged panel (E1) includes DAPI counterstaining, single channel panels (E2-4) are color-coded. E. Compared adult expression of mCherry with the RG marker GS (green) and the neuronal marker HuC/D (blue) in her5actswitch fish. The mCherry polyclones include ventricular RG (small green arrows point to RG cell bodies) and neurons. F. Compared expression of mCherry with PCNA-positive proliferating progenitors, showing that mCherry polyclones contribute to the TPZ (yellow arrows to the TPZ, white arrowheads to the PML) with high magnifications of the boxed area in panel F1. Scale bars: A2-D2 100 µm, A3-D3 50 µm, E,F 20 µm. Abbreviations: Cb: cerebellum, TeO: tectum opticum, TPZ: tectal proliferation zone, TsC: torus semicircularis. |
|
her4-positive RG exhibit neurogenic activity in the post-embryonic TeO. Compared fate in the 5mpf TeO of RG cells expressing her4 at 1.5mpf, assessed by Cre-mediated tracing (A) and rtTA-mediated birthdating (B). Panels 1. Cross-sections of a TeO hemisphere immunostained for mCherry and GS and counterstained with DAPI and observed in confocal microscopy. Panels 2–4. High magnifications of the areas boxed in panels 1 (single and merged channels as indicated, maximum projection of 4 sections over 3 µm). In both cases, a single stripe of mCherry-positive progeny cells is observed, identically located along the antero-posterior and dorso-ventral axes and comprising both neurons and RG. Scale bars: A1 and B1 100 µm, A2-A4 and B2-B4 50 µm. |
|
Post-embryonic her4-positive tectal RG are neurogenic during a brief window of competence linked with their position close to the TPZ. A-D. Compared distributions in the adult TeO of the progeny of her4- (A1, A2) and her5- (B1, B2) positive cells, traced from 1dpf (A1, B1) or 5dpf (A2, B2) by means of Cre-mediated recombination in her4actswith and her5actswith animals. Confocal images of horizontal sections at equivalent dorso-ventral positions, anterior left, immunostained for mCherry expression and counterstained with DAPI. Red arrows in A1, A2 point to the posterior limit of the her4-derived mCherry-positive stripe. Pink arrows in B1, B2 point to the anterior limit of the her5-derived mCherry-positive domain. Note that the position of these limits recedes towards posterior as recombination is induced at later stages, and that the her4-derived stripe is consistently anterior to the her5-derived domain. C1 and C2 are interpretative drawing (color-coded, see key) of superimposed her4actswitch and her5actswitch recombinations at 1dpf and 5dpf, respectively. D. Top panel: 9TB induction and analysis time points for the her4H2a-mCherry animals photographed in D1-D4. Bottom panels: Whole-mount dorsal views of TeOs in adult her4H2a-mCherry fish induced between 2 dpf and 1.5mpf, observed for H2a-mCherry expression under a fluorescence binocular. TeO surrounded by dotted line, anterior left, arrows to the H2a-mCherry-positive stripes. Note the receding position of these stripes with later induction time points. E. Compared antero-posterior positions in the adult TeO of an her4actswith, T(5dpf) -recombined stripe (red) and BrdU-positive cells pulsed at 5dpf (green). Yellow arrow to the identical location of the two stripes. Horizontal section observed by confocal microscopy, immunostained for mCherry and BrdU, and counterstained with DAPI. F. Antero-posterior position in the adult TeO of BrdU-positive cells pulsed at 15dpf (green). Horizontal section observed by confocal microscopy, immunostained for BrdU and counterstained with DAPI. Note the coincidence with the anterior limit of the her5actswith,T(5dpf) domain (pink arrow in panel B2). G. High magnification of a her4actswith, T(5dpf) -recombined stripe in the adult TeO, co-immunostained for mCherry and GS and counterstained with DAPI. Confocal views of a horizontal section, anterior left, yellow arrows to the mCherry-positive stripe. G4 is an interpretative drawing of G1 with identical color code. From anterior to posterior: anterior RG cells (yellow asterisk) are not overlaid with mCherry-positive neurons (black asterisk); RG at the level of the stripe (yellow asterisk, yellow arrows in G1) are overlaid with mCherry-positive neurons (red stripe); RG located posterior to the stripe are not recombined (green asterisk). Scale bars: A,B,E,F 100 µm, G 20 µm. |
|
Evidence for a minor direct route of TeO neurons generation independent of her4-positive RG progenitors. A. Organization of polyclones at the TPZ border in her4actswith, T(5dpf) adults, co-immunostained for mCherry and PCNA and counterstained with DAPI. High magnification of a horizontal section, confocal microscopy. A1: merge; A2, 3: single channels. Note that polyclones extending across the entire PGZ are attached to the most ventricular domain of the TPZ. B, C. Compared fate of her4-positive and BrdU-labelled progenitors in the adult TeO. Top panel: experimental scheme. B. Low magnification of a horizontal section showing an entire TeO hemisphere in a double labelled adult, co-immunostained for mCherry, BrdU and GS, and counterstained with DAPI. C. High magnification views of the area boxed in B, located anterior to the labelled stripe. C1: merge, C1-C3: single channels. Confocal views. Note that recombination of the RG layer anterior to the stripe is complete. D. High magnification views of the stripe area, as boxed in B. D1: merge, D2-D4: single channels. Confocal views. Yellow arrows point to double mCherry and BrdU-positive neurons, green arrow to neurons positive for BrdU only. Scale bars: A, B 100 µm, C, D 50 µm. |
|
The sequence of progenitor subtypes at the lateral pallial edge is compatible with a reiteration of the TeO sequence. Compared expression of Her4-GFP, GS and PCNA (A) and her9, Her4-GFP and PCNA (B) in triple labelled cross-sections of the adult pallium, counterstained with DAPI. her9 expression is revealed by fluorescent in situ hybridization, and all other markers by immunocytochemistry. Confocal views. Panels 2–5 are high magnifications views of the boxed areas in panels 1. Panels 1 is the merged view, panels 3–5 are single channels. Red and green arrows in A3, A4 point to the lateral limit of the GS- and Her4-GFP-positive zones, respectively. The red arrowhead in B3 points to the medial limit of the her9-positive domain. Scale bars: 50 µm. C. Interpretative drawing showing the relative positions of the her9, PCNA, her4 and GS-positive zones. This organization is reminiscent of the TeO neurogenic sequence. Progression from a lateral pool to RG was shown in Dirian et al., (2014). |
|
Construction and expression profile of Tg(her5:mCherry) compared with the expression of other transgenic reporters, genes and cell type markers in the adult midbrain. A. Schematic of the genomic area covered by the her5-containing BAC (pTARBAC2.1) used for transgenesis. The her5 locus is magnified in green (exons) and white (untranslated areas), and red domains depicts the start and stop codons. The mCherry or Ert2CreErt2 cassettes (red box) were inserted in frame within the second exon of her5. The entire BAC was flanked with Tol2 sites and used for transgenic generation, these sequences coupled with a cmlc2:GFP cardiac cassette (blue and green box) were inserted at the loxP511 locus. B-D. Compared reporters expression in Tg(her5:eGFP) (Tallafuss and Bally-Cuif, 2003), Tg(her5:mCherry) (this study) and Tg(her4:eGFP) (Yeo et al., 2007) transgenic lines in the adult midbrain. Cross sections at the level indicated on the schematics, counterstained with DAPI; arrows point to expression sites. E-G. In situ hybridization for the endogenous genes (blue signal, blue arrows) in the different transgenic lines. H,I. Compared expression of Her5- mCherry with PCNA on horizontal (H) and sagittal (I) sections (planes indicated on the schematics) of an adult midbrain observed in confocal microscopy. Panels 2: low magnification views, with merged fluorescence channels and counterstained with DAPI. Panels 3-5: high magnifications of the area boxed in panels 2, channels/stainings colorcoded. J, K. Compared expression of Her5-mCherry with the neuronal marker HuC/D (J) and the glial marker GFAP (K), respectively, on cross sections of an adult midbrain observed in confocal microscopy. Panel 3-4: High magnifications of the area boxed in the schematics, with channels/stainings color-coded. White arrowheads to the PML on panels H,I5 and J,K4. Scale bars: B-G 50μm, H,I 100μm, J-K 10μm. Abbreviations: TPZ: tectal proliferation zone. |
|
Validation of the Tg(her5:CreERT2) transgenic line. A-D. Compared expression of endogenous her5 and cre mRNA revealed by in situ hybridization on whole-mount Tg(her5:CreERT2) transgenic embryos at 24hpf (A,B) and 2 dpf (C,D). Arrowheads to the PML. E. Compared expression of mCherry, GS and HuC/D in a crosssection of the TeO area of a her5actswitch,T(2dpf) larva analysed at 5dpf (section plane indicated on scheme). Confocal view. mCherry expression is limited to the PML (compare with Fig.2D). F. Polyclones issued from the mosaic recombination of her5actswitch,T(1dpf) embryos observed on cross-sections of the TeO at 5dpf (section plane indicated on scheme) and analysed for the glial marker GS (F3) and the neuronal marker HuC/D (F4). Confocal view. F2-F5 are high magnifications (single and merge fluorescence channels, color-coded) of the polyclone boxed in F1. Note the tight association between mCherry-positive neurons and RG (F5, purple and yellow arrows, respectively). Arrowheads to the PML in F1. G-I. Polyclones issued from the mosaic recombination of her5actswitch,T(1dpf) embryos observed on horizontal sections of the adult TeO in three different animals. Note the common anterior limit of their distribution, but that the spatial distribution of these polyclones within each mCherrypositive territory varies from fish to fish. Scale bars: E and F1 20μm, F2-F4 10μm, G-I 100μm. Abbreviations: TeO: tectum opticum. |
|
Validation of the Tg(her4:ERT2CreERT2) line for faithful recombination in the post-embryonic midbrain. A-F. Compared expression of endogenous her4 and cre revealed by in situ hybridization on whole-mount embryos at 24hpf (A,B), whole-mount dissected 5dpf brains (C,D, dorsal views anterior left) and cross-sectioned 5dpf midbrains (E,F). G. Compared expression of mCherry recombined in her4actswitch,T(5-7dpf) larvae and analysed at 7dpf, with expression of the glial marker GS and the neuronal marker HuC/D. Confocal views of cross sections at the level indicated on the schematic. Note that mCherry expression is faithfully restricted to tectal RG (G4), a location identical to endogenous her4 expression (compare with F). H, I. Validation of the Tet-On approach. H. Expression of endogenous her4 revealed by in situ hybridization in a 15dpf brain. I1-3. High magnification of the area boxed in H. Compared expression of H2A-mCherry and GS in a her4H2a-mCherry,9BT(15dpf) animal analysed one day post treatment (ie at 16 dpf). Confocal views. I1-I2 are single channels (color-coded), and I3 is a merged view with DAPI counterstaining. Note the coincidence of H2A-mCherry and RG in the TeO (arrowheads in I3). Note also that induction faithfully reflects endogenous her4 expression (compare with H). Scale bars: G 5μm , I 50μm. Abbreviations: TeO: tectum opticum. |
|
High magnification in the adult TeO of transversal polyclones generated from her4-positive RG at 5dpf and 15dpf, revealed using the TetOn system in her4H2a-mCherry, T(5dpf) (A) and her4H2a-mCherry, T(15dpf) (B) fish induced with 9TB. Horizontal sections stained for H2A-mCherry and the RG marker GS, and counterstained with DAPI (Panels 1 and 2: single channels, panels 3: merge), observed under confocal microscopy. Note that these polyclones cover the entire transversal extent of the PGZ and include RG cells (yellow arrows). Scale bars: 50μm. |
Reprinted from Developmental Biology, 420(1), Galant, S., Furlan, G., Coolen, M., Dirian, L., Foucher, I., Bally-Cuif, L., Embryonic origin and lineage hierarchies of the neural progenitor subtypes building the zebrafish adult midbrain, 120-135, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.