- Title
-
The mediator complex subunit Med10 regulates heart valve formation in zebrafish by controlling Tbx2b-mediated Has2 expression and cardiac jelly formation
- Authors
- Just, S., Hirth, S., Berger, I.M., Fishman, M.C., Rottbauer, W.
- Source
- Full text @ Biochem. Biophys. Res. Commun.
|
Effects of the png mutation on heart development. (A-D) Wild-type (wt) (A) and png mutant (C) embryos and close-ups of the wt (B) and png (D) hearts at 72 hpf. (E, G) MF20 and S46 immunostainings reveal that chamber specification and differentiation is not altered in png mutants (E). (F, H) H&E staining of sections of wt (F) and png (H) hearts at 72 hpf. png mutant heart chambers are not separated by an AV ring and no endocardial cushions (EC) develop at the AVC, whereas png myocardial (my) and endocardial (en) cell layers are clearly defined. (I, J) anf is misexpressed in AV myocardial cells of png hearts (I), whereas anf is absent from wt AV myocardium (J) (AVC, white arrow). (K, L) Spp1 is expressed in AVC and OFT endocardium of wt embryos at 72 hpf (K), whereas spp1 expression is absent from the AVC of png hearts (L) (Schematic descriptions: I′, J′, K′, L′). (M) Integrated genetic and physical map of the png locus on chromosome 19. The png promoter mutation (1193bp insertion) is indicated (red). (N) The png mutation leads to severely diminished med10 transcription (relative expression 0.074 ± 0.039, n = 7, P < 0.001), whereas mRNA levels of UBE2QL1 are unaltered (relative expression 0.93 ± 0.18, n = 3, ns). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Defective Tbx2b and not Wnt or Nodal signaling accounts for defective AVC development in png. (A, A′, B, B′) Heat-shock induced overexpression of Wnt8 to activate canonical Wnt signaling using the transgenic line Tg(hsp70l:wnt8a-GFP). Overexpression of wnt8 in wt does not phenocopy the png heart phenotype (n = 35, 3 independent experiments). (C, D) Ectopic expression of ndr2 in png mutants cannot rescue the heart phenotype (n = 29, 3 independent experiments). (E) Efficient expression of myc-tagged ndr2 in png mutants was demonstrated by Western-Blot. (F-I) In contrast to wt (F, H), tbx2b staining is absent in AVC of png mutants at 72 hpf (G, I). (J, L) tbx2b expression is reconstituted in med10 RNA-injected png mutants (L), but not in controls (J). (K, M) Accordingly, anf is misexpressed in AV myocardial cells of KCl-injected png mutants (K), whereas anf is absent from rescued AV myocardium of med10 RNA-injected png mutants (M) (white arrow, AVC). EXPRESSION / LABELING:
|
|
Ectopic expression of Tbx2b rescues the valve defect in png mutants. (A-E) Injection of tbx2b RNA into homozygous png mutants leads to the reconstitution of endocardial cushion development (19.70% ± 2.32%, n = 101, P < 0.001, 4 independent experiments) (B-D), whereas injection of KCl has no effect (A). (D) H&E staining of sections of a tbx2b RNA-injected png heart at 72 hpf. Endocardial cushion development (black arrow) in png hearts is reconstituted. (E) In tbx2b RNA-injected png hearts, anf is only present in the ventricular and atrial myocardium and not in the AVC. (B, F-H) Injection of foxn4-RNA into homozygous png mutants does not rescue endocardial cushion development (n = 87, 3 independent experiments). (G) H&E staining of sections of a foxn4 RNA-injected png heart at 72 hpf. No endocardial cushions are formed in foxn4 RNA-injected png hearts. (H) Tbx2b expression is not reconstituted in AV myocardial cells in png mutants injected with foxn4-RNA. (I, J) Injection of MO-foxn4 leads to a heart valve phenotype in 81.80% ± 10.33% (n = 122, 4 independent experiments) and co-injection of med10-RNA and MO-foxn4 does not rescue the foxn4 morphant phenotype (80.01% ± 5.36, n = 198, 4 independent experiments). (K, L) Injection of MO-foxn4 in wt (31.31% ± 28.61%, n = 54, 4 independent experiments) and heterozygous png embryos (56.79% ± 31.36%, n = 97, 4 independent experiments) revealed a sensitizing effect of Foxn4-depletion on heterozygous png embryos. PHENOTYPE:
|
|
Defective Has2 signaling in png mutant hearts. (A-D) has2 staining is absent in AVC and OFT endocardium of png mutants at 48 (B) and 72 hpf (D). (E, F) Histological sections of wt (E) and png mutants (F) at 72 hpf stained for hyaluronic acid (HA) (brown) and counterstained with H&E. The amount of HA in the cardiac jelly of png mutants is severely reduced. EXPRESSION / LABELING:
PHENOTYPE:
|
|
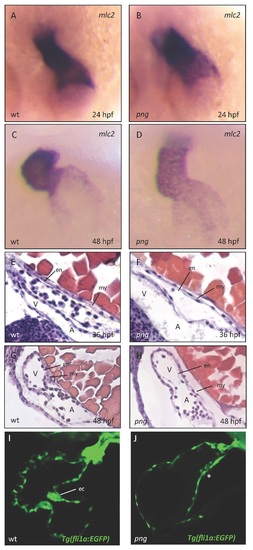
Figure S1: Early cardiac development proceeds normally in png mutant embryos. Similar to wild-types (A, C), png mutant embryos (B, D) show a normal distribution of cmlc2 RNA at 24 and 48 hpf as revealed by whole-mount antisense RNA in situ hybridization. The heart regularly jogs to the left and loops to the right in wild-type (A, C) and png mutant (B, D) zebrafish embryos. (E-H) Hematoxylin/Eosin staining of sagittal histological sections of wt (E, G) and png mutant (F, H) hearts at 36 and 48 hpf, respectively. Similar to the wild-type situation (E, G), png mutant hearts (F, H) consist of normally developed endo- and myocardial cell layers. (I, J) Confocal images of Tg(fli1a:EGFP) transgenic wt (I) and png mutant (J) hearts at 72 hpf, showing that endocardial cushion cells fail to cluster at the AVC in png mutant hearts (marked with *). A, atrium; V, ventricle; my, myocardium; en, endocardium; ec, endocardial cushions. PHENOTYPE:
|
|
Figure S2: Defective myo- and endocardial patterning in png. (A, B) In contrast to the wild-type (A), bmp4 staining is observed throughout the myocardial layer of the png atrium and ventricle (B) and is not restricted to the inflow tract (IFT), AVC and OFT myocardium. (C, D) Endocardial notch1b expression is enhanced at the AVC and OFT of wild-type embryos (C), but is diffusely expanded throughout the atrial and ventricular endocardium in png mutant embryos (D). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Figure S3: Ectopic expression of Med10 rescues png mutant embryos, whereas knockdown of zmed10 phenocopies the png mutant phenotype. (A-D) Ectopic expression of wild-type med10 RNA can rescue the heart phenotype of a significant proportion of png mutant embryos (84.5 ± 4.2%, n = 85, 3 independent experiments) (B, C), whereas KCl control-injection has no effect (A, C). (D) Hematoxylin/Eosin staining of sagittal histological sections of a med10 RNA-injected png mutant heart at 72 hpf. Endocardial cushion development (black arrow) in png mutant hearts is reconstituted. (E-G) Knockdown of zmed10 by injection of Morpholino-modified antisense oligonucleotides (MO-med10) phenocopies the png mutant phenotype (MO1-med10: 98.37% ± 0.45%, n = 240, 3 independent experiments; MO2-med10: 95.53% ± 0.68%, n = 185, 3 independent experiments) (E, G), whereas injection of the same amount of standard control Morpholino-modified antisense oligonucleotides (MO-control) does not impact on AV canal development (F, G). PHENOTYPE:
|
|
Figure S4: (A, B) Transgenic zebrafish Tg(hsp70l:wnt8a-GFP) at 24 hpf, expressing wnt8a-GFP under the control of the heat shock inducible hsp70l promoter. Heat shock efficiently induces the expression wnt8a-GFP. (C-F) Incubation of png embryos with 0.1 M LiCl to activate Wnt signaling does not sensitize heterozygous png embryos to develop the png heart phenotype (n = 34, 3 independent experiments). |
|
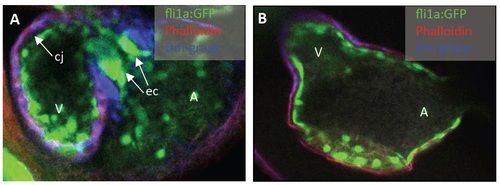
Figure S5: (A, B) Confocal sections of Rhodamine-Phalloidin- (red, indicating myocardium) and DM-Grasp (Alcam; blue, indicating myocardium and differentiated AV endocardial cells) stained wt (A) and png mutant (B) transgenic Tg(fli1a:EGFP) (GFP expressed in all endothelial cells) zebrafish embryos, at 72 hpf. In png mutants (B), no differentiated AV endocardial cells can be detected and cardiac jelly is markedly reduced compared to wt controls (A). PHENOTYPE:
|