- Title
-
Developmental suppression of schizophrenia-associated miR-137 alters sensorimotor function in zebrafish
- Authors
- Giacomotto, J., Carroll, A.P., Rinkwitz, S., Mowry, B., Cairns, M.J., Becker, T.S.
- Source
- Full text @ Transl Psychiatry
|
Generation and validation of molecular tools used to transgenically inhibit or overexpress miR-137. (a) Transgene used to ubiquitously express anti-miR137 sponges (named βactin:mCherry:10 × SP137). (b) Transgene used to overexpress synthetic-miR137 (named UAS:YFPs:miR137). (c) Validation of synthetic-miR137 and anti-miR137 sponge transgenic expression/activity in 3 dpf zebrafish. Transgenic animals βactin:mCherry:10 × SP137, which ubiquitously express mCherry:10 × SP137, were injected with two plasmids simultaneously (i) 503UNC:Gal4 expressing Gal4 specifically in muscle cells and (ii) UAS:YFPs:miR137 expressing YFP fused to synthetic-miR137. Muscle-specific expression of synthetic-miR137 induced by presence of Gal4 can be tracked by the presence of YFP (processed in green in the present pictures), and correlates with downregulation of mCherry fluorescent intensity, confirming efficient activity of both anti-miR137 sponges and synthetic-miR137. Mosaic expression of YFPs:miR137 was observed as the transgenes were injected and thus not stably integrated into the genome. (d) Confocal images (0.86-µm section) showing endogenous miR-137 translational repression activity on a transcript carrying anti-miR137 sponges in 3-dpf zebrafish. The injected zebrafish (βactin:mCherry:10 × SP137; SEN:GFP) expressed mCherry:10 × SP137 ubiquitously and GFP specifically in sensory neurons (Rohon–Beard cells presented in c. These neurons also expressed endogenous miR-137 (Supplementary Figures 1 and 5). Due to the presence of miR-137, mCherry:10 × SP137 transcript translation was repressed, resulting in poor mCherry expression. Compared with MO-control, injection of MO137-02 (8 ng) dramatically increased fluorescent intensity, confirming that 8 ng MO137-02 was sufficient to inhibit endogenous miR-137 activity (quantification are presented in Supplementary Figure 5). GFP, green fluorescent protein; YFP, yellow fluorescent protein. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Transient manipulation of miR-137 activity. (a) Design of morpholinos (MO) targeting the three dre-pre-miR137 copies, with mature miR-137 highlighted in yellow. Alignment was performed using CLUSTAL 2.1. (b) (i) Morphology of 72 hpf zebrafish embryos injected with MOs. (b) (ii) Morphology of 72 hpf zebrafish embryos injected with synthetic miRNAs. (c-h) Percentage of dead, malformed and normal embryos at 28 hpf following injections of MOs and synthetics miRNAs. MO137-02, MO-Control and miR-control injections were well-tolerated, while MO137-01 and miR137-mimics injections induced strong morphological defects that were most likely due to off-specific effect (see Results). These malformations included, but were not limited to, (1) heart edema, (2) curved tail and (3) abnormal trunk curvature. Percentage of normal embryos is presented in white, abnormal in light gray and dead in dark grey. (i-j) In situ hybridization against dre-miR137 at 3 dpf showing that 8 ng of MO137-02 inhibits endogenous miR-137 expression in zebrafish to a threshold that is no longer detectable even after extended revelation. miR, miRNA. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
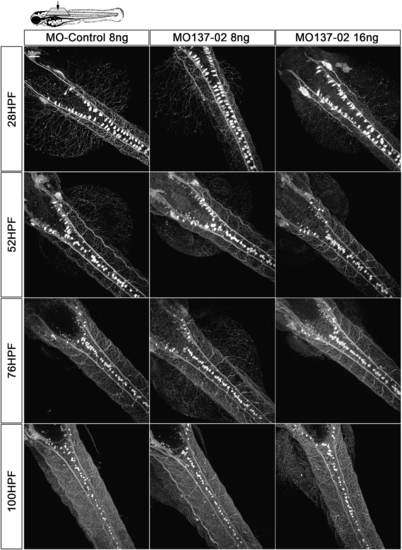
miR-137 knockdown does not impair Rohon–Beard- (RB-), dorsal root ganglia- (DRG-), trigeminal- or Mauthner-(M-)neurons differentiation and seems to not modify their overall network. SEN:GFP transgenic animals were injected with 8–16 ng of MO-Control or MO137-02 to observe RB, M and DRG cells in vivo. No significant difference with regard to the number of cells was observed between the different conditions (Counting available in Supplementary Figure 7). No obvious difference was observed in terms of axonal network, but an in-depth analysis should be performed to conclude. Following anti-GFP immunostaining, 10 animals per conditions were analyzed at different time points using confocal microscopy and image analysis. Brightness of the original image was enhanced. GFP, green fluorescent protein; MO, morpholino. |
|
dre-miR-137 in situ hybridisation. A-C, in situ hybridisation using an LNA probe on 72hpf zebrafish embryos. miR-137 is expressed in the nervous system including in fore-, mid- and hindbrain. miR-137 is also highly expressed in sensory neurons (white arrows). |
|
miR-137 knockdown does not impact overall motor neuron development. A transgenic zebrafish line available in our laboratory (MN:GFP) that expresses GFP fluorescent protein into motor neurons was injected with 8ng of MO-Control or MO137-02. No significant difference was observed between the different conditions. |
|
miR-137 knockdown does not impact overall vascular system development. Fli1:GFP zebrafish (4dpf) expressing GFP into the vascular system was injected or not with 8ng of MO-Control or MO137-02. No significant difference was observed between the different conditions. |