- Title
-
Polq-Mediated End Joining Is Essential for Surviving DNA Double-Strand Breaks during Early Zebrafish Development
- Authors
- Thyme, S.B., Schier, A.F.
- Source
- Full text @ Cell Rep.
|
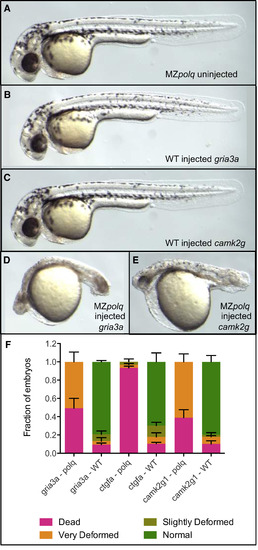
Polq Is Required for Survival following Cas9-Induced DNA DSBs All images are of∼36 hr post-fertilization (hpf) embryos. Additional images are available in Figure S2. Sequences of the three gRNAs used are available in Table S1. (A) Homozygous MZpolq mutant. (B) WT embryo injected with Cas9 protein and gRNA for the gria3a gene. (D) [sic] WT embryo injected with Cas9 protein and gRNA for the camk2g gene. (D) MZpolq mutant injected with Cas9 protein and gRNA for the gria3a gene. (E) MZpolq mutant injected with Cas9 protein and gRNA for the camk2g gene. (F) Survival rates and fraction of deformed embryos observed for MZpolq and wild-type embryos injected with Cas9 protein and gRNAs targeting one of three genes. Embryos were injected at the one-cell stage, and all data were collected at ∼36 hpf. Depictions of the observed deformities are available in Figure 1 and Figures S2A–S2C. The experiment was performed twice, with n = 15–47 for each injection. See also Figure S2 and Table S1. |
|
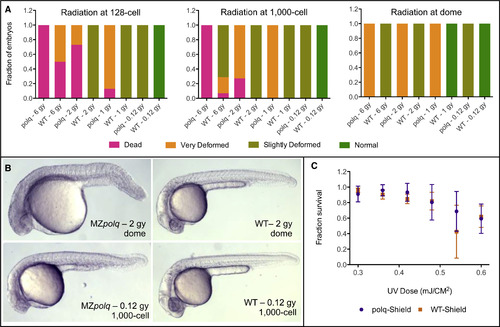
Figure 2. Polq Is Required for Survival following DSBs Induced by Ionizing Radiation (A) Survival rates and fraction of deformed embryos observed for MZpolq and WT embryos exposed to varying levels of ionizing radiation at three stages of early development (n = 14–32 for each condition). These data were collected at ∼24 hpf. (B) Examples of observed deformities in MZpolq embryos compared with wild-type embryos subjected to the same levels of radiation. Additional examples are available in Figure S2D. (C) Survival rate observed for MZpolq and wild-type embryos exposed to varying levels of UV radiation at the shield stage of development. The experiment was performed two to four times per condition, with n = 14–54 each time. See also Figure S2. PHENOTYPE:
|
|
Related to Figure 1. Additional images of injected MZpolq mutant embryos. a) MZpolq mutant embryos injected with gRNA only look normal. Embryos injected with Cas9 protein (three examples, “Cas9 only”) show slight deformities. These three embryos would be considered “slightly deformed”, rather than “very deformed”. b and c) Three additional examples each of MZ-Polq mutants injected with Cas9 protein and gRNA for camk2g (b) and gria3a (c) are shown, all of which would be considered “very deformed”. d) Examples of MZpolq and wild-type embryos exposed to varying levels of ionizing radiation at multiple developmental stages. |
|
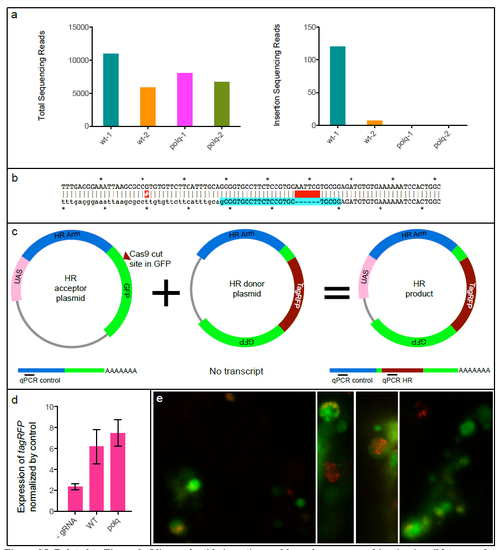
Related to Figure 3. Oligonucleotide insertion and homologous recombination in wild type and MZpolq embryos. a) Total number of sequencing reads and number of reads containing an insertion for oligonucleotide-injected wild-type (wt) and MZpolq mutant embryos. b) Sequence of the insertion. The resulting allele (top) is an in-frame insertion of a six base-pair EcoRI site (GAATTC) at the camk2g locus (bottom). No basepairs were lost or gained outside of the insertion in this experiment. A single-nucleotide polymorphism between the zebrafish used and reference sequence is annoted with a red-highlighted #. c) Schematic of assay used to quantitatively assess homologous recombination. Homologous recombination generates fusion gene of UAS and tagRFP. Injection of mRNA encoding GAL4 generates GAL4 protein, which binds to UAS and activates transcription. Transcripts are detected by qRT-PCR. Approximate locations of qPCR primers are shown for the transcripts produced from each plasmid. d) Expression of tagRFP mRNA normalized to expression of homology arm RNA. Homologous recombination is stimulated by Cas9-induced DSBs in both MZpolq mutant and wild-type embryos. e) Example images of MZpolq mutant cells in which homologous recombination events occurred as detected by tagRFP fluorescence. |