- Title
-
Osterix/sp7 limits cranial bone initiation sites and is required for formation of sutures
- Authors
- Kague, E., Roy, P., Asselin, G., Hu, G., Stanley, A., Albertson, C., Simonet, J., Fisher, S.
- Source
- Full text @ Dev. Biol.
|
Zebrafish sp7 mutants have delayed early bone development. (A) Sp7 protein sequences of human, mouse, medaka and zebrafish were aligned using ClustalW. Identical residues are shaded dark gray. Cysteine and histidine residues corresponding to the zinc finger domains from positions 318 to 402 (black line) are highly conserved in a domain showing only three amino acid variations between mammals and fish. The mutation p.Leu145* (black triangle) introduces a stop codon, deleting over half of the Sp7 coding sequence, including the three zinc finger domains. (B and C). Calcified bones were detected at 4 dpf by vital Alizarin red staining. While WT (B) and mutant (C) larvae were indistinguishable by morphology, the cleithrum (arrows) and opercle (asterisks) are slightly smaller in sp7 mutants. In situ hybridization was performed for markers of osteoblast differentiation in embryos at 4 dpf. Expression of all markers in the cleithrum was reduced in mutants, especially spp1 and col10a1. |
|
sp7 mutants show progressive skeletal malformations and deficient mineralization. (A-F) Alizarin red stain was performed on sp7 mutants and WT siblings from 2 wpf to 8 wpf. At 2 wpf (A), the skeleton of mutants shows an overall delay in mineralization. The vertebral column is unevenly mineralized and the vertebrae weakly stained. At 4 wpf (B), mineralization in the vertebral column is similar in WT and mutant. However, the skulls of mutants start to manifest a domed shape, the parasphenoid is bent (arrow), and the caudal fin lepdotrichia are wavy (dashed arrow). At 6 wpf (C), midface hypoplasia, protruding mandible and domed skull are evident in mutants. (D) Higher magnification shows the differences in size and shape of vertebrae between WT and mutant fish at 6 wpf. Arrow indicates bending of the dorsal spinous process of a single vertebra. (E) Also at 6 wpf, ribs of mutants frequently show evidence of fractures (arrow). (F) At 4 wpf, the pharyngeal teeth in the fifth ceratobranchial are fully mineralized in WT; while present in the mutants, only the tips are mineralized (arrows). (G and H) Transmission electron microscopy was performed on parietal bones at 6 wpf. (G) The nucleus (Nu) of a normal mature osteoblast is elongated and filled with dense material. In sp7 mutants the nuclei are rounder and filled with a less dense material. The extracellular matrix of mutant bone contains empty vacuoles (v), not observed in WT. (H) Needle-shaped hydroxyapatite crystals are present inside extracellular matrix vesicles in WT bone (arrow), but not observed in sp7 mutants. Scale bars for A-F are 5 mm, and for G and H are 500 µm and 100 µm, respectively. PHENOTYPE:
|
|
Midface hypoplasia correlates with deformation of the parasphenoid. (A) 12 landmarks from the lateral view of the craniofacial skeleton of 6 wpf fish were used for morphometric analyses; representative sp7 mutant and WT fish are shown. (B) Principal component analysis separated sp7 homozygotes into a unique group (green). Heterozygotes group (blue) overlap with WT fish (red), although they are more variable (C) Alizarin red stained mutants representing progressive stages of craniofacial. development are shown in lateral view. Increasingly severe deformation of the parasphenoid is accompanied by midface hypoplasia. We quantified midface hypoplasia as the angle between the parasphenoid (dashed line drawn from the base of the parasphenoid to the anterior of the maxilla) and frontal bones (dashed line from the maxilla to frontal bones). In WT the angle remains nearly constant during development (data not shown), while in sp7 mutants the angle increases (indicated at the bottom left of each picture). Asterisks indicate protrusion of the mandible as a consequence of midface hypoplasia. To quantify the deformation of the parasphenoid, we drew a line from the caudal end of the parasphenoid to its anterior (blue line) and measured the greatest distance to the bone from this line (perpendicular blue line). Note that the distance increases with the severity of hypoplasia observed (indicated at the bottom left in blue). Scale bar is 1 mm. PHENOTYPE:
|
|
Multiple skull bones in sp7 mutants arise from independent ossification sites. (A) Schematic diagram of the skull showing the flat bones (frontal and parietal), the sutures and the cartilage bars. And alizarin red stained dissected skulls from WT and sp7 mutant fish at 6 wpf. In WT, the vault of the skull is made up of paired frontal and parietal bones that overlap by ~6 weeks to form the interfrontal, coronal, and sagittal sutures. sp7 mutants have multiple irregular bones that fail to overlap and form normal sutures. (B and C) We followed skull formation dynamically by imaging individual Runx2:egfp transgenic fish every 2 days. In a WT fish (B), the frontal and parietal bones normally form as single ossifications. The position where the initiation site grows from is indicated (asterisks). In an sp7 mutant (C), additional ossification sites arise laterally (arrows) and grow apically, giving rise to separate bones. Three initiation sites are shown with asterisks. To examine initiation of the frontal bone, we imaged transgenic fish from the earliest appearance of precursor cells. The taenia marginalis is positioned on the top of the figures (D-G). In fish doubly transgenic for a cartilage marker (col1a1:egfp) and Runx2:mch, we first observe precursor cells closely associated with the taenia marginalis anterior cartilage, just posterior to the epiphyseal bar, dashed square area (D). In WT fish (E), the cells expand in 6 days from a thin line to outline the wedge-shaped frontal bone (arrowheads). In sp7 mutants over the same period (F), the cells instead cluster along the cartilage, and stay more closely associated during early growth. G and H illustrate from two different fish the genesis of ectopic bones (arrowheads), starting from small clusters of cells adjacent to the initial ossifications. Scale bars for A-C are 1 mm, and for D–H 0.5 mm. Abbreviations: coronal (c), epiphyseal bar (eb), eye (e), frontal (f), interfrontal (if), parietal (p), sagittal (s), taenia marginalis anterior (tma), tania marginals posterior (tmp). PHENOTYPE:
|
|
Sutures of sp7 mutants show abnormal architecture and gene expression. (A-D) Tissue morphology was assessed by hematoxylin-eosin staining of sagittal sections of the skull to show the coronal suture. In WT (A), suture formation is divided into three progressive phases as the osteogenic fronts (*) approach and overlap: initiation (~4 weeks), approximation (~5 weeks), and overlapping (>6 weeks). In sp7 mutants (B), initiation and approximation phases are similar to WT, but overlapping does not occur, and osteogenic fronts have a rounded shape. During the same period, the frontal bone above the epiphyseal bar cartilage (eb) increases in thickness (C), but in sp7 mutants (D) the frontal bones are thinner (brackets in C and D), and extra sutures are common (arrows). (E-I) Tissue sections from transgenic fish were analyzed by immunohistochemistry for GFP, mCherry, and phosphohistone H3. In WT fish (E), expression of the Runx2:egfp transgene is confined to cells at the osteogenic front, while sp7:mcherry expression is maintained in cells along the bone; both transgenes are expressed at the osteogenic fronts (asterisks). Immature osteoblasts expressing Runx2 (GFP+) are observed at the osteogenic fronts of WT fish (F) while in mutants these cells are not limited to the fronts (G). Proliferation was analyzed in WT (H) and sp7 mutant (I) fish. Phosphohistone H3+cells were more frequently observed at the osteogenic fronts in mutants, and these corresponded to the GFP+ early osteoblasts (merge). (J and K) Expression of msxd (J) at the coronal suture and scales (K) was examined by in situ hybridization in WT and sp7-/-; expression was significantly increased in mesenchymal cells of mutants. (L and M) To examine the level of BMP signaling, sections were analyzed with an antibody to phosphorylated Smad 1/4/5. Numbers of pSmad+ nuclei in and around the suture were much greater in sp7 mutants (M). Scale bars for all panels are 50 µm. Abbreviations: epiphyseal bar cartilage (eb), frontal bone (f), parietal bone (p), scale (s). PHENOTYPE:
|
|
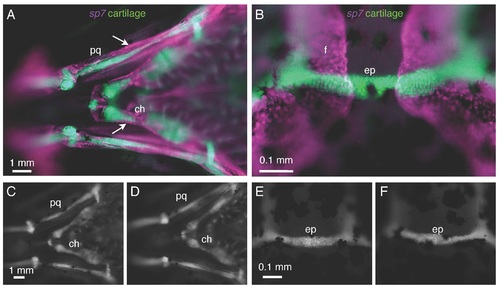
Cartilage does not express sp7 and is not affected in sp7 mutants. A, B) In a 3 wpf fish doubly transgenic for sp7:mCherry and col1a1:egfp, there is no overlap in expression of the two transgenes. A) Perichondral cells surrounding the palatoquadrate (pq) and ceratohyal (ch) cartilages (arrows) express the sp7 transgene. B) Similarly, the epiphyseal bar cartilage (ep) and the frontal bones (f) show no overlap in transgene expression. C-F) At 3 wpf, col1a1:egfp transgenics show no significant differences in cartilage formation or morphology between WT fish (C,E) and sp7 mutant (D,F). Scale bars are 1 mm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
sp7 mutant skulls are phenotypically variable. A-E) Skulls of sp7 mutants at 8 wpf were fixed, stained with Alizarin red, and dissected for analysis. Phenotypic severity varies from a mildly affected skull (A) to almost completely unmineralized skull (E). Extra sutures are present in all fish (arrows in A-E), and patches of unmineralized bone are commonly observed (asterisks in B-D). F) in a senescent fish (>2.5 years), decreased mineralization is observed in flat bones (lighter coloration). PHENOTYPE:
|
|
Misregulation of msxd in scales of sp7 mutants. A-D) In fish transgenic for Runx2:egfp and sp7:mch, GFP+ cells are confined to the distal tip of the scale (arrow in A), while mCh is expressed in those cells as well as more broadly along the scale (B). E, F) While in situ hybridization for msxd reveals little scale expression in WT fish (E), it shows dramatic upregulation in sp7 mutants (F), suggesting a role for Sp7 in regulating BMP signaling during scale growth. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 413(2), Kague, E., Roy, P., Asselin, G., Hu, G., Stanley, A., Albertson, C., Simonet, J., Fisher, S., Osterix/sp7 limits cranial bone initiation sites and is required for formation of sutures, 160-72, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.