- Title
-
Loss of function of myosin chaperones triggers Hsf1-mediated transcriptional response in skeletal muscle cells
- Authors
- Etard, C., Armant, O., Roostalu, U., Gourain, V., Ferg, M., Strähle, U.
- Source
- Full text @ Genome Biol.
|
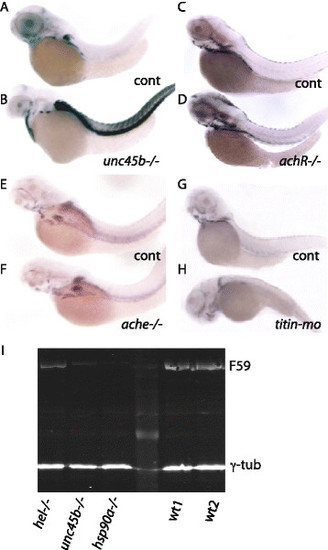
Expression of unc45b mRNA in muscle mutants. unc45b mRNA expression in wild-type sibling (a) and unc45b mutant (b) embryos. unc45b mRNA expression in wild-type sibling (c) and sop mutant (d) embryos with a defective delta subunit of acetylcholine receptor. unc45b mRNA expression in wild-type sibling (e) and ache mutant (f) embryos encoding a defective acetylcholine esterase. unc45b mRNA expression in control (g) and titin morphant (h) embryos. With the exception of unc45b mutants (b) with a myosin folding defect, none of the other mutants with impaired muscle function (d, f, h) showed up-regulation of unc45b mRNA. Embryos were hybridized to unc45b antisense RNA. All embryos are 72 h old; anterior left, dorsal up. i Myosin content in different mutants compared with wild type. Western blot done with protein extracts from embryos 72 hours post-fertilization: titin mutant (hel-/-), unc45b mutant (unc45b-/-), hsp90a mutant (hsp90a-/-) and wild type (WT). Antibodies: F59 recognizing slow myosin, and γ-tubulin as a loading control |
|
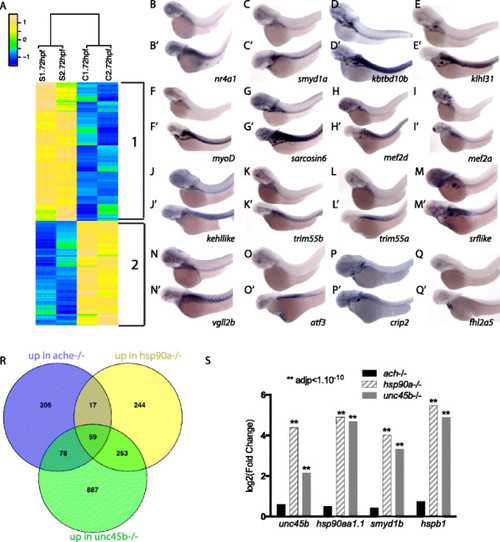
Transcriptome analysis of unc45b mutants. a Hierarchical clustering of genes significantly up-regulated (yellow) or down-regulated (blue) (fold change of at least 1.5-fold, p adj < 0.05) in two independent mRNA samples from unc45b -/- embryos (S1 and S2) relative to two mRNA preparations from wild-type siblings (C1 and C2) at 72 hpf. The relative expression scale is indicated as normalized expression. Both up-regulated genes (851 genes, cluster 1) and down-regulated genes (560 genes, cluster 2) are shown. The relative expression scale is indicated as normalized expression (blue indicates low expression, green moderate expression, and yellow high expression). b–q′ RNA in situ hybridization to verify RNAseq data. Genes targeted by the antisense probes are indicated in the panels: wild-type siblings (b–q), unc45b mutant embryos (b′–q′). All embryos are 72 h old. Anterior left, dorsal up. r Venn diagram of the genes upregulated in ache, para or unc45b mutants at 72 hpf. Note that in this case DESeq2 was used for the detection of up-regulated genes in order to increase the power of the differential expression analysis. s Log2 fold change of unc45b, hsp90a, smyd1b and hspb1 obtained by RNAseq in 72-hpf ache-/-, unc45b-/- and hsp90a-/-. Asterisks above the bars indicate significant adjusted p values (adjp) |
|
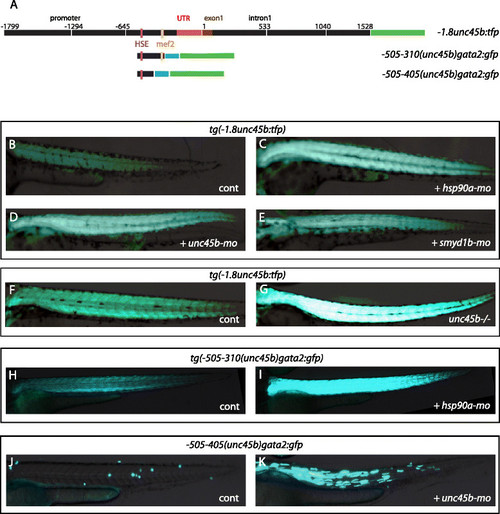
unc45-derived transgenes phenocopy the response to misfolded myosin. a Scheme representing the 3.3-kb sequence of the unc45b gene that recapitulates muscle-specific expression (-1.8unc45b:tfp) and its derivatives -505/-310(unc45b)gata2:gfp (Figure S3c in Additional file 8, construct 16) and -505/-405(unc45b)gata2:gfp (Figure S3i in Additional file 8, construct 31). All positions are indicted relative to the A of the ATG start codon (+1) of unc45b. Red bars represent the untranslated region (UTR). The first exon is indicated in brown. Blue bars represent the gata2 minimal promoter. Green bars represent TFP or green fluorescent protein (GFP) reporter genes. The pink vertical bar indicates the heat shock element (HSE); the yellow vertical bar indicates the Mef2 binding motif. b–e Deficiency in myosin folding activates the -1.8unc45b:tfp construct. Tg(-1.8unc45b:tfp) embryos injected with either hsp90a-mo (c), unc45b-mo (d) or smyd1b-mo (e) show an increase of TFP compared with the uninjected control (b). f, g In comparison with transgenic wild-type sibling embryos (f), expression of Tg(-1.8unc45b:tfp) in unc45b mutant embryos (g) is elevated. This confirms the results from the morpholino knock-down experiments (b, d). h, iTg(-505/-310(unc45b)gata2:gfp) embryos injected with hsp90a-mo (i) show an increase of GFP expression compared with the uninjected control (h). j, k Embryos injected with the construct -505/-405(unc45b)gata2:gfp show no GFP expression (j). However, co-injection with unc45b-mo triggers GFP expression in skeletal muscle (k). Thus, this transgene containing only 100 bp of the unc45b upstream region from -505 to -405 lost the basal muscle expression but retained the response to misfolded myosin. All embryos are 72 h old; anterior left, dorsal up |
|
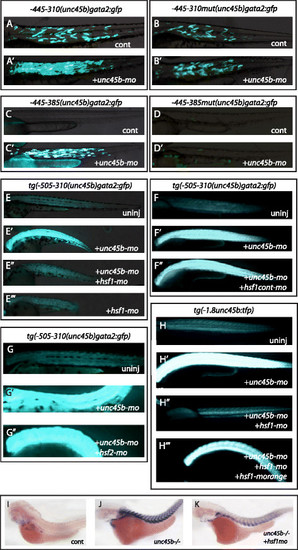
Hsf1 mediates the response to misfolded myosin. a, a′ Co-injection of unc45b-mo with the construct -445/-310(unc45b)gata2:gfp (Figure S3f in Additional file 8, construct 25) leads to an increase of GFP expression (a′) compared with embryos injected with the plasmid alone (a). b, b′ Co-injection of unc45b-mo with the construct -445/-310mut(unc45b)gata2:gfp in which the Hsf1 binding site was destroyed by four point mutations (Figure S3f in Additional file 8, construct 26) does not show an increase of GFP (b′) compared with embryos injected with the transgene alone (b). The basal muscle expression was unaffected by the point mutations. Thus, the Hsf1 recognition sequence is important for the transgene′s response in embryos with a myosin folding defect but not for basal expression in the muscle cells. c, c′ The -445/-385(unc45b)gata2:gfp construct (Figure S3i in Additional file 8, construct 35) lacking the Mef2 binding site but containing the Hsf1 recognition sequence does not drive any GFP expression when injected into wild-type embryos (c). However, co-injection of unc45b-mo with this transgene triggers activation of GFP expression in muscle cells (c′). d, d′ -445/-385mut(unc45b)gata2:gfp (Figure S3i in Additional file 8, construct 42) carries point mutations in the Hsf1 binding site in addition to a deletion of the Mef2 binding site. This construct does not show GFP expression when injected alone (d) or in combination with unc45b-mo (d′). Thus, in this construct both the basal expression in muscle cells and the misfolded myosin response are abolished. e-e′′′ Knock-down of Hsf1 (hsf1-mo) abolished the misfolded myosin response. Transgenic embryos stably expressing Tg(-505/-310(unc45b)gata2:gfp) were either not injected (e) (basal muscle expression) or injected with unc45b morpholinos (e′) (unc45b-mo, misfolded myosin induced expression) or double injected with morpholinos (e′′) directed against unc45b and hsf1 (hsf1-mo) or with hsf1-mo alone (e′′′). Co-injection of hsf1-mo and unc45b-mo (e′′) blocked the induction of the transgene as observed by injection of unc45b alone (e′). Injection of hsf1-mo alone (e′′′) (compare with (e′′) or (e)) did not alter basal muscle expression, demonstrating that Hsf1 is only required for the misfolded myosin response and not for basal muscle expression of the transgene. f-f′′ Injection of a hsf1cont-mo harboring five mismatches does not prevent the ability of -505/-310(unc45b)gata2:gfp to respond to the accumulation of unfolded myosin. Tg(-505/-310 (unc45b)gata2:gfp) was either not injected (f), or injected with the unc45b-mo (f′), or unc45b-mo and hsf1cont-mo together (f′′). Expression of GFP reporter in double injected embryos (f′′) is as high as in embryos injected with unc45b-mo alone (f′). g-g′′ Knock-down of Hsf2 (hsf2-mo) does not impair the response of Tg(-505/-310(unc45b)gata2:gfp) to misfolded myosin. Tg(-505/-310(unc45b)gata2:gfp) embryos were either not injected (g) or injected with unc45b-mo (g′), or unc45b-mo and hsf2-mo (g′′). h-h′′′ Co-injection of the plasmid encoding Hsf1-mOrange fusion protein rescued the misfolded myosin response. Tg(-1.8unc45b:tfp) embryos were either not injected (h), or injected with unc45b-mo (h′) or with unc45b-mo and hsf1-mo (h′′) or with unc45b-mo, hsf1-mo and hsf1-morange (h′′′). The triple-injected embryos showed TFP reporter expression (h′′′) comparable to that of embryos injected with unc45b-mo alone (h′). i-k Knock-down of Hsf1 (hsf1-mo) reduced the expression of unc45b mRNA from the endogenous gene in unc45b mutants. In situ hybridization with unc45b probe on either wild-type embryos (i), unc45b mutants (j), or unc45b mutants injected with hsf1-mo (k). unc45b mutants were unequivocally identified by the lack of well-formed myofibrils and the total lack of motility. All embryos are 72 h old and are shown anterior left and dorsal up |
|
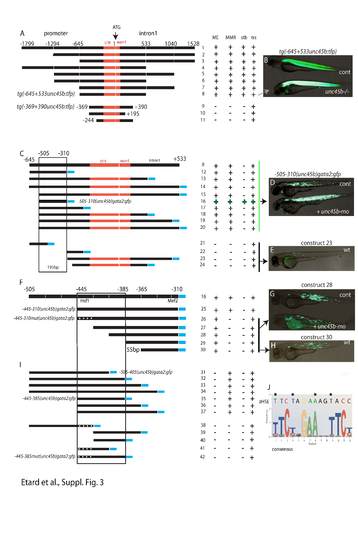
Summary of the transgenes used to map the regulatory elements mediating the misfolded myosin response A-B: tg(-1.8unc45b:tfp) (“1”) and its deletion derivatives were expressed transiently (trs) or as transgenesstably integrated into the genome (stb). By serial deletion analysis 645 bp upstream of the ATG of unc45bwas identified to still drive basal muscle expression and respond to unc45b deficiency (B). Furthertruncation of the unc45b sequences to the region from -369 bp to +390 bp abolished the response.C-E: Refined analysis of the unc45b regulatory sequences contained in tg(-645/+533unc45b:tfp). Theunc45b deletion fragments were cloned in front of the gata2 promoter (blue bars). Transgenes containingthe 195 bp fragment (“16”, D) harboring sequences from -505 to -310 drove GFP expression in skeletalmuscle (D, cont) and reacted to the accumulation of unfolded myosin (D, +unc45b-mo). Deletion constructs12-20 retained basal muscle expression and responded to misfolded myosin. In contrast, constructs 21-24give no GFP expression (see E).Further mutations of the 195 bp unc45b fragment separates the regulatory sequences responsible for basalmuscle expression and the response to misfolded myosin. Construct 28 drove basal GFP expression inskeletal muscles but did not respond when co-injected with the unc45b-mo (G). Construct 30 (55 bp)reacted similarly (H). These constructs retained a binding site of the Mef2 transcription factor. Constructs 31-37 lack the muscle basal expression but still respond to misfolded myosin (I). Thus, the region from -445 to -425 is important to mediate the response. This region contains a homology to the heat shock responseelement (HSE, J). The HSE was mutated by introduction of 4 point mutations (asterisks J, F, I) in constructs “26”, “38”, “41” and “42”. Mutation of the HSE leads to loss of the misfolded myosin response. Abbreviations:ME: muscle expression. The numbers above constructs indicate position relative to the ATG of unc45b. |
|
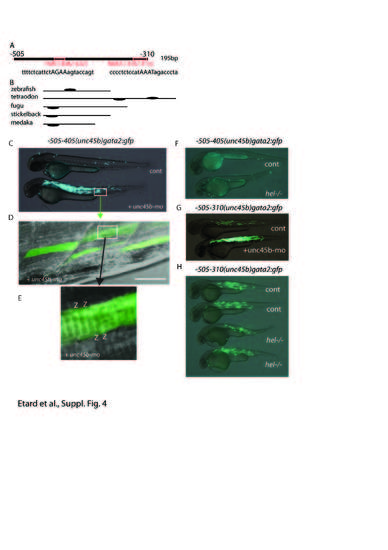
a, b Sequence comparison of the unc45b region mediating the misfolded myosin response with homologous regions in the unc45b genes of other fish species. a Scheme of the 195-bp zebrafish unc45b fragment (-505 to -310 relative to the ATG) containing the HSE at position -446/-422 and the Mef2 binding site at position -335/-313. The recognition sequence is depicted below each site. b Comparison of the zebrafish unc45b sequence from -505 to -310 with regions in the unc45b genes of four other fish species revealed the presence of a conserved HSE element (black ovals). c–e Injection of -505/-405(unc45b)gata2:gfp plasmid into wild-type embryos (cont) (c) do not generate any GFP expression, whereas co-injection with unc45b-mo activates the regulatory sequence (c) (unc45b-mo). Examination of the GFP fibrils revealed classic striations (d, e, z) (Z-line). f injection of -505/-405(unc45b)gata2:gfp plasmid into hel mutant (hel/) or wild-type siblings (cont) do not lead to GFP0 expression. g, h Injection of -505/-310(unc45b)gata2:gfp plasmid into wild type (cont) (g, h), unc45b morphant (unc45b-mo) (g) or hel mutants (hel/) (h) show a GFP upregulation in unc45b morphant but not in hel mutants. |
|
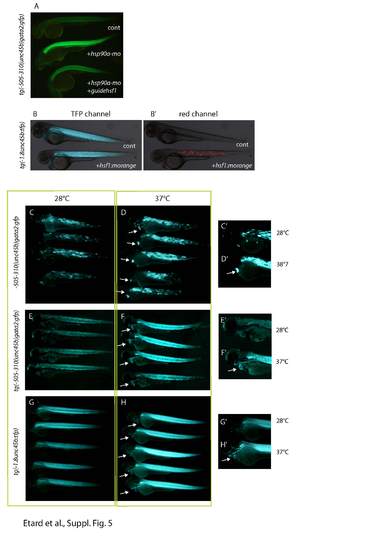
aTg(-505/-310 (unc45b)gata2:gfp) embryos were injected with hsp90a morpholinos (hsp90a-mo) or with hsp90a-mo together with a mix of CRISPR RNA directed against hsf1 and cas9 mRNA (hsp90a-mo + guide hsf1). We note a decrease of GFP in the hsf1/hsp90a double knock-down compared with the hsp90a single morphant. b-b′ Injection of the plasmid encoding a Hsf1-mOrange fusion protein does not activate the unc45b promoter. Expression of TFP reporter in hsf1-morange injected embryos (+hsf1:morange) (a, b) is as high as in uninjected tg(1.8unc45b:tfp) embryos (cont) (a). c-h′ Heat shock triggers unc45b up-regulation. -505/-310(unc45b)gata2:gfp injected embryos (c, d, c′, d′), Tg(-505/-310 (unc45b)gata2:gfp) (e, f, e′, f′) and tg(-1.8unc45b:tfp) (g, h, g′, h′) transgenic embryos were raised at 28 °C until 48 hpf and heat shock at 37 °C for 12 hours (d, d′, f, f′, h, h′). Embryos shown in (c, c′, e, e, g, g′) were kept at 28 °C. The heat shock embryos show up-regulation of GFP in skeletal and cardiac muscles, demonstrating that the regulatory sequences of unc45b react to heat shock. |