- Title
-
NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Authors
- Dona, M., Bachmann-Gagescu, R., Texier, Y., Toedt, G., Hetterschijt, L., Tonnaer, E.L., Peters, T.A., van Beersum, S.E., Bergboer, J.G., Horn, N., de Vrieze, E., Slijkerman, R.W., van Reeuwijk, J., Flik, G., Keunen, J.E., Ueffing, M., Gibson, T.J., Roepman, R., Boldt, K., Kremer, H., van Wijk, E.
- Source
- Full text @ PLoS Genet.
|
Morphological, functional and epistatic effects of ninl and dzank1 knockdown in zebrafish retina. (a-c) Images of 4 dpf living zebrafish. Un-injected controls (WT) appear morphologically normal (a), while embryos injected with 2 ng of ninl atgMO display morphological defects, including ventrally curved body axis and small eyes (b). Embryos injected with 6 ng dzank1 ex8 spMO resulted in expanded melanophores, small eyes and severe pericardial edema (c) (a'-c') Retinal histology of 4 dpf zebrafish morphants examined by cryo-sections, where bodipy highlights the OS (red) and nuclei are stained with DAPI (blue) in all panels. Outer segments were shortened and dysmorphic in ninl and dzank1 morphants compared to wildtype larvae. (d-g) Ninl interacts genetically with dzank1. Injection of sub-effective dzank1 (1 ng/nl) MO (e') or ninl (0.5 ng/nl) MO (f') shows normal OS shape and length in morphologically normal appearing larvae, which could not be distinguished from un-injected embryos (WT) or control MO-injected larvae (d'). Combined injection of sub-effective concentrations of ninl (0.5 ng/nl) and dzank1 (1 ng/nl) MO together results in almost complete absence of OS (g'). (h) Quantification of Outer Segment length, shown as a scatter graph where each datapoint represents the mean OS length in one larva, revealed a significantly decreased length of outer segments in ninl (2ng/nl), dzank1 (6ng/nl) and ninl/dzank1 double morphants as compared to controls. Bars represent the mean value for each treatment group with the Standard error of the mean (SEM) ***P<0.0001, ** P<0.001, unpaired Student's t-test. (i) Analysis of the Opto Kinetic Response (OKR) showing severely decreased responses in larvae injected with 2 ng ninl atgMO or 6 ng dzank1 ex8 spMO (*** p<0.0001, Student's t-test). Scale bars represent 500 μm (a-c and e-h) and 15 μm (a'-c' and e'-h'). RPE, retinal pigment epithelium; OS, Outer Segment; IS, Inner Segment; ONL, Outer Nuclear Layer; OPL, Outer Plexiform Layer. PHENOTYPE:
|
|
dzank1 and ninl knockdown leads to accumulation of vesicles and vacuoles in zebrafish photoreceptors. Transmission electron microscopy of control (a), ninl knockdown larvae (b,d) and dzank1 knockdown larvae (c,e). Ninl and dzank1 morphants (b-e) demonstrate absent or shortened and dysmorphic outer segments (OS), whereas connecting cilia (CC) are still intact (arrowheads in b-c,h), and accumulation of vesicular structures (v), highlighted in the boxed area in e (e'). Sub-effective concentration of ninl (f) and dzank1 (g) MO-injection leads to a normal phenotype compared to control MO-injected larvae (a) and uninjected controls. Injection of combined sub-effective concentrations of dzank1 and ninl (h) MO leads to increased vesicle accumulation in OS (h') and inner segment (h''). (i) Analysis of the presence of vesicles in inner segments of photoreceptor of cells (%). On the Y—axis the different classes are indicated (minimum of 6 eyes per group). (* P<0.01 and *** P<0.001 Student's t-test). Larvae in all panels are 4 dpf. Scale bars represent 0.5 μm. OS: outer segment, CC: connecting cilium, m: mitochondria, n: nucleus, v: vesicular structures, G: Golgi system, L: lysosome. PHENOTYPE:
|
|
NINL and DZANK1 associate specifically with complementary subunits of the cytoplasmic dynein 1 motor complex. (a) Strep-SILAC and TAP experiments show that DZANK1 interacts specifically with DYNLL1 and DYNLL2 (yellow). NINL associates with components of the dynactin complex (red) and with most components of the dynein 1 motor complex (blue), except for DYNLL1 and DYNLL2. The solid line between NINL and DZANK1 symbolizes a direct interaction, whereas the dashed lines stand for interactions determined by immune and affinity purifications. (b-g'') Genetic interaction between ninl and dync1h1. MO-injection with a high concentration of dync1h1 (3 ng/nl) shows larvae with pericardial edema, small eyes and short OS (d-d''). MO-injection of sub-effective concentrations of ninl (0.5 ng/nl) (e-e'') or dync1h1 (1 ng/nl) (f-f'') generates larvae with a normal phenotype and normal OS length, compared to wildtype larvae (b-b'') and control MO-injected larvae (c-c''). The combination of sub-effective MO concentrations of ninl (0.5 ng/nl) and dync1h1 (1 ng/nl) results in short larvae and virtual absence of outer segments (g-g''). All larvae are 4 dpf. Panels b'-d'' and e'-g'' are retinal cryo-sections stained with bodipy (red) to highlight the outer segments and DAPI to stain the nuclei. (h) Quantification of photoreceptor outer segment length revealed a significantly decreased length of outer segments in dync1h1 (3ng/nl) and dync1h1/ninl double morphants as compared to controls (*** P<0.0001; two-tailed, unpaired Student's t-test). Scale bars represent 500 μm (b-g), 50 μm (b'-g') and 15 μm (b''-g''). PHENOTYPE:
|
|
(a-d) Overview of Ninl and Ush2a localization in cryo-sections of 4 dpf zebrafish retina using anti-Ninl (red signal), anti-Ush2a (green signal) and the basal body marker anti-Centrin (cyanid signal). In all images the nuclei are counterstained with DAPI (blue signal). (a-a''''') Un-injected wildtype, (b-b''''') 10 ng/nl control MO-injected, (c-c''''') 2 ng/nl ninl MO-injected and (d-d''''') 6 ng/nl dzank1 MO-injected zebrafish retinas. (a'-d') Anti-Ush2a staining (green signal) is strongly reduced in dzank1 and ninl morphants (c'-d'), while Ush2a is clearly present in wildtype and control MO-injected larvae. (a''-d''). Specific Ninl-immunofluorescence (red signal) was largely abolished in ninl morphants and reduced in dzank1 morphants. (a'''-d''') Centrin (cyanide signal) was observed in wildtype, un-injected control and in both ninl and dzank1 morphants. (a''''-d'''') Co-localization of Ush2a and Ninl (yellow signal) was observed in wildtype and control MO-injected larvae. (a'''''-d''''') Co-localization of Ush2a and Centrin (yellow signal) was seen in all images (WT, Control Oligo, ninl and dzank1 morphants), despite strong reduction of Ush2a immunofluorescence in ninl and dzank1 morphants. Scale bars represent 15 μm, except for (a-d) in which the scale bars represent 50 μm. |
|
ninl and dzank1 knockdown results in epinephrine-induced melanosome retraction delay. Control MO-injected larvae (10 ng/nl; n = 20) at 5 dpf (a-a'''), ninl morphant (2 ng/nl; n = 20) (b-b''') and dzank1 morphant (6 ng/nl; n = 20) (c-c'''). White Box denotes the area at higher magnification (40x) (a'-c''). (a-c') Melanosome pattern of the different larvae before treatment, (a''-c''), 5 min after epinephrine addition and (a'''-c''') 20 min after epinephrine addition, t represents time in minutes. (d) Graphical representation with error bars (standard deviation) demonstrating a significant delay of epinephrine-induced melanosome retrograde trafficking compared with wild-type and control MO-injected (10 ng/nl) larvae. Treatment (control, ninl- or dzank1-morphants) is noted on the x-axis and average response time in minutes is noted on the Y-axis. ***: P<0.001 (two-tailed, unpaired Student's t-test). PHENOTYPE:
|
|
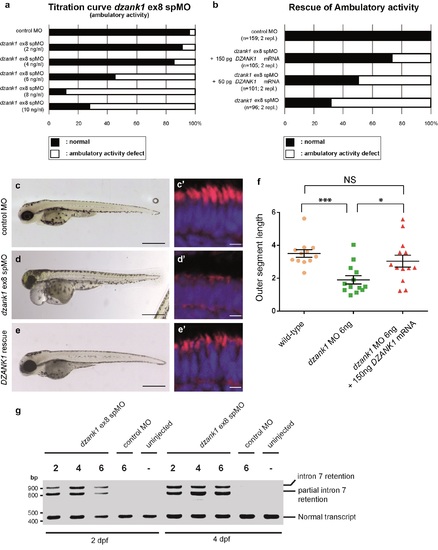
Specificity of the dzank1 ex8 spMO. Titration curve of the dzank1 ex8 spMO scored on ambulatory activity shows an increased incidence of the phenotype with an increasing dose (a). (b) Co-injection of 6 ng dzank1 ex8 spMO with 150 pg capped MO-resistant mRNA encoding human DZANK1 reduced the incidence of phenotypes including ambulatory activity, small eyes (c, d, e) (n>95/group, p<0.0001 (two-tailed Fisher's exact test) and restored photoreceptor outer segment lengths (n = 13, P<0.001 (two-tailed, unpaired Student's t-test), c', d', e', f). (f) Quantification of photoreceptor outer segment lengths revealed a significant increase in length in the DZANK1 rescue group (3.0+/-0.4 μm) as compared to dzank1 morphants (6ng/nl; 1.9+/-0.25 μm) (P<0.001; two-tailed, unpaired Student's t-test). Bars indicate mean OS length per group and Standard error of the mean (SEM) (g) Characterization of the effect of the dzank1 ex8 spMO at 2 and 4 dpf by RT-PCR analysis. Injection of various amounts of MO resulted in the (partial) retention of intron7, leading to a premature termination of translation. PCR fragments were analyzed by Sanger sequencing. Scale bars represent 500 μm (c-e) and 15 μm (c'-e'). PHENOTYPE:
|
|
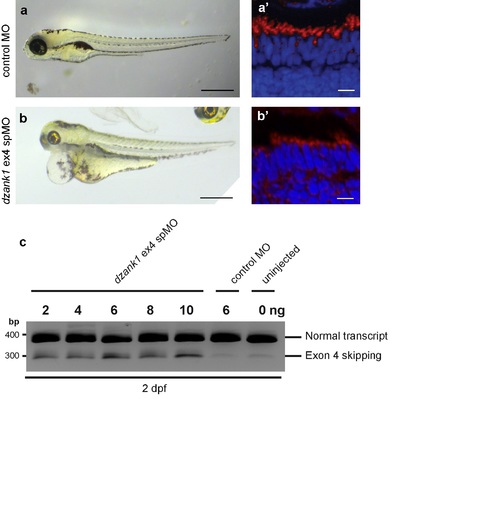
Recapitulation of the phenotype by a second dzank1 spMO targeting exon4. Injection of a dzank1 ex4 spMO-injected larvae completely recapitulates the phenotype observed in dzank1 ex8 spMO-injected larvae, including pericardial edema, small eyes, defects in ambulatory activity (b) and shortened photoreceptor outer segments (b') as compared to control MO-injected larvae from the same clutch (a,a'). (c) Characterization of the effect of the dzank1 ex4 spMO at 2 dpf by non-quantitative RT-PCR analysis. Injection of various amounts of MO resulted in the (partial) skipping of exon4, leading to a premature termination of translation. PCR fragments were analyzed by Sanger sequencing. Scale bars represent 500 μm (a-b) and 15 μm (a'-b'). PHENOTYPE:
|
|
Ninl or Dzank1 knockdown in zebrafish leads to mis-localization of rhodopsin. Immunofluorescence with anti-opsin antibody 4D2 demonstrated mis-localization of opsins (indicated by arrows) in the photoreceptor cell body in ninl (b-b') and dzank1 (c-c') morphants, compared to controls, where opsins are restricted to the outer segments (a-a'). (a'-c') are the white boxed areas of (a-c). Larvae are 4 dpf. Scale bars represent 50 μm (a-c) and 15 μm (a'-c'). |