- Title
-
Pronephric tubule morphogenesis in zebrafish depends on Mnx mediated repression of irx1b within the intermediate mesoderm
- Authors
- Ott, E., Wendik, B., Srivastava, M., Pacho, F., Töchterle, S., Salvenmoser, W., Meyer, D.
- Source
- Full text @ Dev. Biol.
|
Distinct expression patterns for mnx2b and mnx1 in intermediate mesoderm. (A-F) Flat-mounts of embryos co-stained for mnx genes (blue) and papc (brown). Expression of mnx2b (A-C) and mnx1 (d-F) in the IM is present in 2 somite (A, D), 5 somite (B, E) and 10 somite stage (C,F) embryos. Black arrows point to the gata1 signal, black arrowheads indicate the lateral edge of mnx staining, white arrowheads the medial edge of mnx1 staining. Note that anterior mnx1 signals at 5 and 10 somite stage forms a lateral (black arrowhead) and a medial stripe (white arrowhead). Yellow boxes indicate positions of higher resolution images shown in G-J. (G-J) Double-ISH stainings for mnx genes (blue) with intermediate mesoderm markers (red) for nephrogenic (pax2.1; G-I) and hematopoietic cell lineages (gata1; J, J′). Shown are brightfield (G, H) and fluorescence images (G′-J′). Mnx2b expression is excluded from gata1 labeled hematopoietic regions (arrow in G, G′), but completely overlaps with the pronephric marker pax2.1 (H, H′). In contrast, mnx1 signals are flanking or intermingled with signals for pax2.1 (2 somite stage; I, I′) and gata1 (10 somite stage, J, J′). |
|
Mnx2b knock down causes proximal pronephric tubule dilation and excretion defects. (A, B) Lateral views of 4dpf Tg(cldnb:lynGFP) control (A) and mnx1/mnx2b morpholino injected embryos (single plane confocal image, proximal tubule is indicated by white arrowheads). (C-F) Dorsal views of proximal tubule segments (C, D) as well as proximal to distal transition domains (E, F) in 2dpf Tg(cldnb:lynGFP) control (I, J) and morpholino injected embryos. (G) Tubule width in control, single and combined morpholino injected embryos at 4dpf. (H, I) Excretion of rhodamine dextran after injection into the common cardinal vein in controls (H, H′) and mnx-morphant (I, I′) Tg(cldnb:lynEGFP) embryos at 3dpf (lateral view, white arrows indicate position of duct lumen). 92% of controls showed excretion 5min after injection, compared to only 35% of Mo-mnx injected embryos (P). |
|
Mnx knockdown causes defects in tubular cilia arrangement and epithelial morphology. (A, B) Single plane confocal images showing tubule cilia stained by anti-actelytaled tubulin (red). Shown are lateral views of proximal tubules of control (A, A′) and mnx-morpholino injected Tg(cldnb:lynGFP) embryos (B, B) (C-F) Transverse TEM sections of proximal tubules in control (C, D) and mnx-morpholino embryos (E, F). Note disorganized microvilli structures after mnx gene knockdown (scale bars correspond to 5 µm (C, E) and 2 µm (D, F)). (G-J) Transverse sections of proximal tubules in control and mnx morphant embryos at 2dpf. Staining of baso-lateral surface directed alpha6F (G, H) and apically located Phalloidin (I, J) displayed similar expression patterns in control and MO-mnx. (K, L) Single plane confocal images of distal tubules stained for Phalloidin (red, ventral view). White arrows indicate displaced epithelial cells with unpolarized Phalloidin signals. |
|
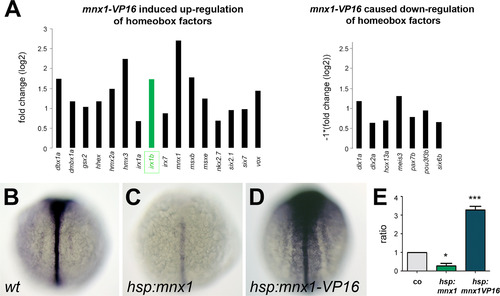
Mnx1 negatively regulates irx1b expression. Microarray analysis identified differential expression of homeobox-containing genes in mnx1-VP16 induced animals compared to wild type controls. Genes attributed with the GO terms transcription regulation and homeobox that have increased expression in mnx1-VP16 animals are indicated (note that the robust mnx1 induction corresponds to the induced mnx1-VP16 transcripts). Shown are genes with >1.5 fold increase or reduction in expression after mnx1-VP16 activation (A). Of three identified irx family genes, irx1b showed the highest change in expression (labeled in green). Whole mount in situ hybridization for irx1b in wild type controls (B), mnx1 induced (C) and mnx1-VP16 induced (D) embryos, dorsal view. Negative regulation of irx1b through Mnx1 was further confirmed in qPCR analysis (E; p val <0.05 (*), <0.001 (***)). |
|
Direct transcriptional repression of irx1b through Mnx factors. Outline of the transplantation experiment. Mnx1VP16 embryos are injected with fluorescein dextran at the 1-cell stage. Fluorescein positive cells are transplanted into wt embryos during shield stage. Embryos are fixed 2h after 1h inductive heat-activation and analysed for fluorescein and irx1b expression (A). Transplanted transgenic cells ectopically express irx1b (B, B′). Lateral view of irx1b stained wt embryos is shown (C) and after CHX treatment (D). Irx1b expression is similarly induced in mnx1-VP16 expressing embryos (E, F), and similarly reduced in mnx1 overexpressing embryos (G, H) without or with CHX, respectively. |
|
Mnx gene knockdown results in posteriorly expanded expression of irx1b. Flat-mount views of wild type and morpholino-injected embryos. Double-ISH analysis for either mnx2b or irx1b (blue) and pax2.1 (brown) shows localization within the nephric mesoderm at the 5som stage (A). Mesoderm expression of irx1b at the 8som stage is posteriorly expanded in mnx morphant embryos (C) compared to controls (B). Arrowheads point to posterior end of irx1b signals. Measurements of the distance from the posterior end of irx1b signals to the tail-bud are presented for single and double morpholino injected embryos (D). |
|
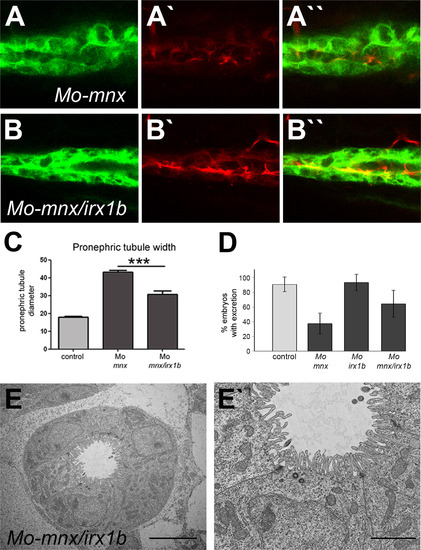
Knockdown of irx1b in mnx morphants partially rescues pronephric phenotypes. Shown is a lateral view of Acetylated tubulin stained Tg(cldnb:lynEGFP) embryos that were injected with Mo-mnx alone (A, A′) or in combination with Mo-irx1b (B, B′). Measurement of tubule widths shows partial rescue of the MO-mnx phenotype in the combination mnx/irx1b double knockdown (C). Excretory defects in Mo-mnx injected embryos are rescued by additional irx1b knockdown (D). TEM sections of embryos co-injected for mnx and irx1b morpholinos demonstrate reduced pronephric tubule width and restored morphology of microvilli (E, E′; Scale bars correspond to 5 and 2 µm, respectively). |
|
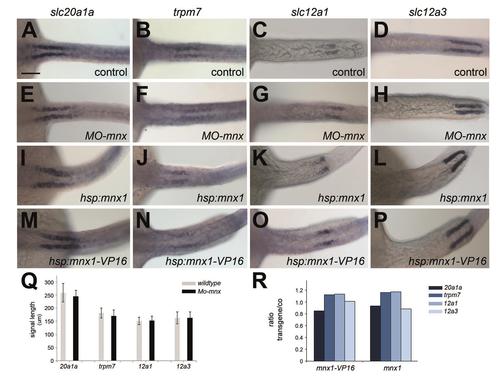
Pronephric tubule patterning is independent of Mnx function. Expression of slc20a1a (A, E, I, M), trpm7 (B, F, J, N), slc12a1 (C, G, K, O) and slc12a3 (D, H, L, P). (A-H) Similar expression patterns of proximal and distal nephron segment markers in uninjected and mnx1/mnx2b-Morpholino (MO-mnx) injected embryos at 48hpf. (I-P) Expression and positioning of segment markers in hsp:mnx1 and hsp:mnx1-VP16 induced embryos is similar to controls. Body axis shortening after heat-induction at the 2 somite stage was observed in both transgenic lines. (Q, R) Signal-to-bodylength ratios of Mo-mnx injected or transgenic induced animals did not show significant differences. |
|
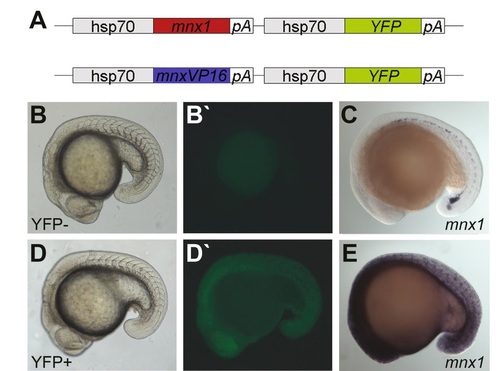
Conditional activation/repression of Mnx1 target genes. Scheme of constructs used for the generation of mnx1 inducible transgenic fish lines (A). These transgenic lines contain either mnx1 in its transcriptional repressor form or a VP16-coupled transactivativing form of mnx1. Expression of the Mnx1 transcriptional repressor/activator is induced by the heat-shock promoter hsp70. The co-activation of a fluorescent YFP protein enables verification of efficient transgene activation (B, B′′, D, D′′). Activation of mnx1 was further confirmed through increased mnx1 expression in ISH stainings (C, E). |
|
Tubule dilation in mnx2b-morphants is independent of cardiac defects. Injections of a second mnx2b directed morpholino (MOmnr2b). (A) Proximal tubule dilation is very similar to mnx2b-MO injected embryo. (B-D) The change in kidney formation is independent of heart defects, as heart-beat rate was unaffected (B) and no heart edema were observed in mnx2b morphants (C, D). Shown are bright field (left) und GFP images of corresponding Tg(-8cldnb.1:lynEGFP)zf106 embryos. |
|
Histology of tubule and glomeruli in 3 dpf mnx2b morphants. Histological horizontal section of a pronephric duct (A, B) and of a glomerolus (B, D) in control animal (A, B) and in mnx2b morphant (C, D). Dilated pronephric duct (C) and glomerolus of a mnx2b morphant (D). Note the urinary pole on both glomerula (up). ca = capillary, c = cilia, p = podocyte, pnd = pronephric duct. Scale bar is 50 µm. Rombout fixed and Polybed 812 embedded zebrafish were cut serially with a Histo Butler diamond knife (Diatome, Switzerland) with a thickness of 2 µm, stained with 1% methylen blue and 1% AZUR II in 1% sodium tetraborate for 2 minutes and examined with a leica DM5000B microscope. Images were made with a Leica DFC490 digital camera and aLeica application suite V4 software. |
|
Ciliated cell specification occurs independently of Mnx functions. Expression of multiciliated and transporting epithelial cell markers is shown for 2dpf embryos (ventral view). Multi-ciliated marker shippo1/odf3b (A, C) and mono-ciliated marker trpm7 (B, D) are comparable in controls and mnx morpholino injected embryos. Scale bars correspond to 100µm. Cell numbers are summarized in (E). |
|
Normal cilia structure in mnx2b and irx1b morphants. TEM images of cilia from the posterior part of the pronephric duct of 3dpf embryos showing regular ultrastructure. A: control, B: irx1b-MO, C: mnx2b-MO 1:2, D: mnrx2b-MO + irx1b-MO. Scale bar is 100 nm |
|
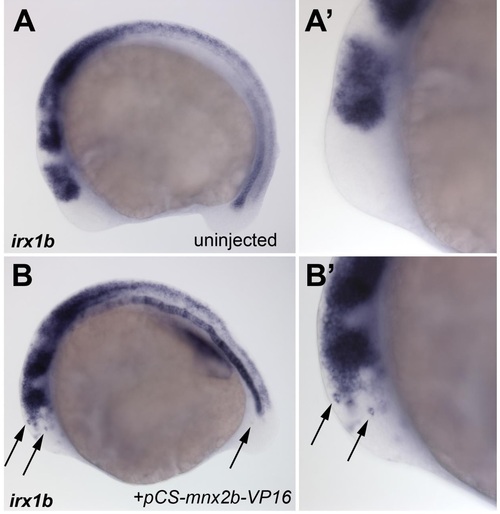
Ectopic induction of irx1b by mnx2b-VP16. Whole mount stains for irx1b expression in a 12 somite stage control embryos (A, A′) and in an embryo injected with 10ng/µl pCS2-mnx2b-VP16 DNA (B,B′). Arrows show individual irx1b positive cells in the brain and tail. A′, B′ show same embryos in higher magnification. |
|
irx1b is expressed in anterior IM domains. In situ hybridization flat mounts of 10 somite stage embryos and lateral views at the 18 somite stage. Irx1a is not found in the IM, but shows pronephric expression at 18 somites (A, B); irx1b is the only irx gene with specific IM expression at somites, but shows no pronephric expression in later stages (C, D); irx3a shows weak expression in ventral domains at 10 somites and no pronephric expression (E, F); irx3b is not present in the IM, but appears in the pronephros at later stages (G, H). Arrows point to mesoderm expression. |
|
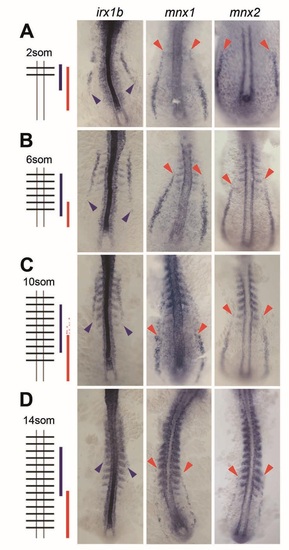
mnx genes and irx1b are complementary expressed during somitogenesis. IM expression of mnx1, mnx2b and irx1b at the 2som (A), 6som (B), 10som (C) and 14som (D) stage in flat-mounted embryos. Staging was confirmed by somite-staining through co-labeling of myoD. Posterior extent of irx1b expression (blue arrowheads) and anterior extent of mnx expression (red arrow heads) are outlined. Additional faint mnx1 signals at the 10som stage are highlighted (red arrows). |
Reprinted from Developmental Biology, 411(1), Ott, E., Wendik, B., Srivastava, M., Pacho, F., Töchterle, S., Salvenmoser, W., Meyer, D., Pronephric tubule morphogenesis in zebrafish depends on Mnx mediated repression of irx1b within the intermediate mesoderm, 101-14, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.