- Title
-
Sonic hedgehog functions upstream of disrupted-in-schizophrenia 1 (disc1): implications for mental illness
- Authors
- Boyd, P.J., Cunliffe, V.T., Roy, S., Wood, J.D.
- Source
- Full text @ Biol. Open
|
disc1 expression is absent in smob641 mutant embryos at 50hpf. Whole mount in situ hybridization analysis for disc1 expression in smob641 (B) revealed a near total loss of expression throughout the CNS and other tissues compared with sibling controls (A). Dorsal views, anterior to left. |
|
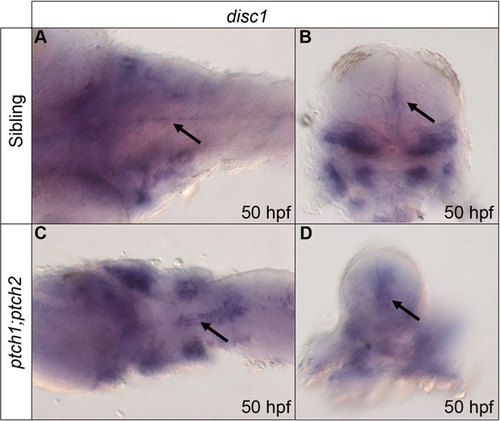
Expression of disc1 at 50hpf is up-regulated around the midline of the hindbrain in response to increased Shh signalling. Expression was analysed using whole mount in situ hybridization. Dorsal, anterior to left (A,C) and transverse sectional (B,D) views show increased disc1 expression around the midline of the hindbrain in ptch1;ptch2 mutants (C,D) compared with siblings (A,B). Arrows indicate hindbrain midline disc1 expression in transverse sectional views. |
|
Reverse-transcription quantitative PCR (RT-qPCR) analysis demonstrates that disc1 expression is altered in sonic hedgehog pathway mutants. (A) Graphical representation demonstrating that expression of disc1 was below the threshold for detection in smob641 mutant embryos at 50hpf. (B) Expression of disc1 was 0.65-fold lower in ptch1;ptch2 double mutants than siblings (P=0.0027). Both ptch1;ptch2 mutant (C; 1.8-fold, P=0.035) and sibling (D; 2.4-fold, P<0.0001) pools show increased disc1 expression relative to smob641 sibling embryos at 50hpf. (n=4; *P<0.05, **P<0.005, ****P<0.0001; error bars show s.e.m.). |
|
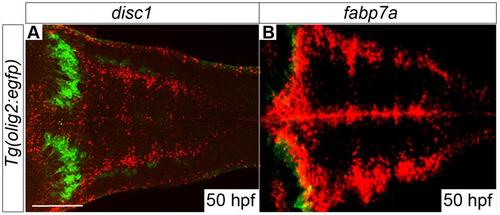
disc1 is expressed in olig2-positive cells located around the midline of the hindbrain at 50hpf. A fluorescent in situ stain for disc1 mRNA (A, red) was performed in tandem with immunofluorescence staining for EGFP (B, green) on fixed Tg(olig2:egfp) embryos. The merged image shows clear overlap of the red and green signals in cells distributed along both sides of the midline of the hindbrain, but not in the abducens motor neurons (arrow). Dorsal views, anterior to left. EXPRESSION / LABELING:
|
|
Cyclopamine treatment leads to a dramatic reduction in the number of olig2-positive cells specified in the hindbrain. Cyclopamine (50µm) was applied to wild type embryos from 32hpf then embryos analysed for olig2 expression at 52hpf. Cyclopamine-treated embryos (B) show a greatly reduced number of olig2-positive cells (arrow) compared with 0.5% ethanol treated (A) and untreated (C) control embryos. n=40, 20 embryos per group, data pooled from 2 independent experiments. |
|
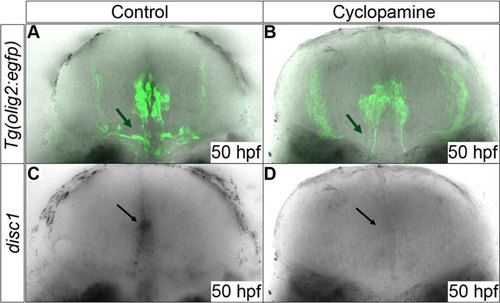
Expression of disc1 in EGFP expressing cells is down-regulated by cyclopamine treatment. Cyclopamine (40µm) was applied to Tg(olig2:egfp) embryos from 34hpf prior to the main period of OPC proliferation and migration. Cyclopamine-treated embryos (D) showed a loss of midline disc1 expression (black arrows) at 50hpf compared to untreated embryos (C). Comparison of B with A shows a loss of EGFP-positive OPCs as a result of chemical inhibition of Shh signal transduction (green arrows). 10 animals were imaged from each group. All images are of transverse sections. |
|
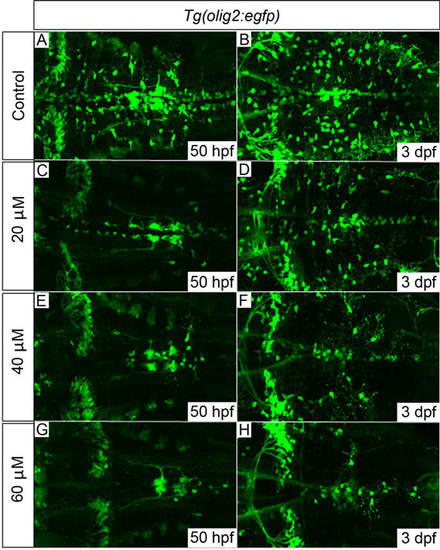
Tg(olig2:egfp) embryos treated with cyclopamine from 34hpf show dose-dependent OPC proliferation and migration defects. OPCs failed to migrate fully to uniformly populate the hindbrain in cyclopamine-treated embryos (C-H) compared with control embryos (A,B). The development of olig2-positive cerebellar neurons (clearly visible towards the left of panel H) and the abducens motor neurons (axonal projections clearly visible in G) appeared normal, even with the highest dose of 60µM, which suggests that their development is largely Shh-independent. 20 embryos per treatment group; images show dorsal views, anterior to left. Quantification of OPC numbers is shown in supplementary material Fig. S5. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of disc1 in dorsolateral regions of the hindbrain resembles fabp7a/blbp expression. Fluorescent in situ hybridisation for disc1 (A, red) and fabp7a/blbp (B, red) was performed on Tg(olig2:EGFP) embryos in conjunction with immunofluorescent staining for EGFP (green) which identifies a population of cerebellar neurones seen as two prominent arcs at superficial levels of the hindbrain. The expression patterns of disc1 and fabp7a appear very similar in dorsolateral regions of the hindbrain. |
|
disc1 expression is greatly reduced in iguts294e mutant embryos at 50 hpf. Whole mount in situ hybridization analysis for disc1 expression in iguts294e (B) revealed a complete loss of midline (arrow in B) and otic vesicle expression compared with sibling controls (A). Transverse sections at the level of rhombomeres 5/6 shown. |
|
OPC distribution is grossly altered in the hindbrain of ptch1;ptch2 mutant embryos. In situ hybridisation for olig2 was performed on ptch1;ptch2 double mutant and sibling embryos fixed at 72 hpf. Dorsal (A, C) and transverse (B, D) views of sibling (A, B) and double mutant (C, D) embryos are shown. In transverse section, no olig2-expressing cells are seen dorsal to the central patch of cells around the midline in wild type embryos (B). In ptch1;ptch2 double mutants the distribution of these cells is greatly altered and cells are seen throughout the dorsal part of the hindbrain (D). |