- Title
-
Solute Carrier Family 26 Member a2 (slc26a2) Regulates Otic Development and Hair Cell Survival in Zebrafish
- Authors
- Liu, F., Xia, W., Hu, J., Wang, Y., Yang, F., Sun, S., Zhang, J., Jiang, N., Wang, H., Tian, W., Wang, X., Ma, D.
- Source
- Full text @ PLoS One
|
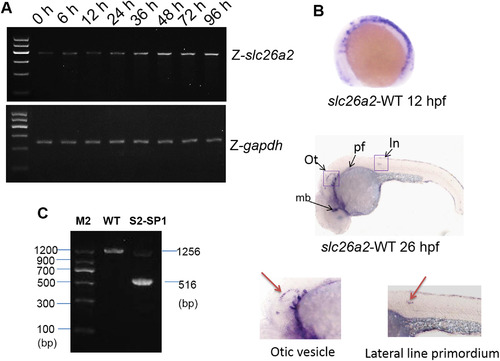
slc26a2 mRNA expression in embryonic and adult zebrafish. (A)RT-PCR analysis of slc26a2 expression in 0–96 h zebrafish yielded 405 bp products, GAPDH served as an internal control for cDNA quantification and gave a 275 bp product. mRNA expression of slc26a2 was detected in the first development period, and transcription remained high after 48 h. (B) Expression of slc26a2 in WT embryos at 12 and 26hpf, detected by in situ hybridization, at 12hpf. The slc26a2 transcript was expressed broadly, and at 26hpf, slc26a2 was expressed circumferentially around the ln, lateral line primordium; ot, otic vesicle; pf, pectoral fin, and mb, midbrain. This suggests a function for slc26a2 in the inner ear and neuromast development. Wild insets depict enlarged images of the otic vesicles and lateral neuromasts. (C) RT-PCR confirmed the effectiveness of S2-SP1, and specific products were amplified from cDNA. Wild-type zebrafish yielded 1256 bp products for slc26a2, and morphants had 516 bp products for S2-SP1 in addition to those observed in WT fish. Two bands appeared for morphants but only one band was seen for WT. |
|
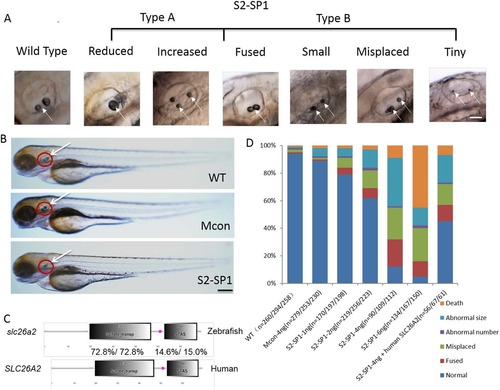
slc26a2 knockdown in wild-type embryos and rescue of slc26a2 overexpression in S2-SP1 morphants. (A) Variations in otolith size of morphant (S2-SP1 knockdown) embryos were observed at 72hpf. Based on the otolith size, malformed embryos were classified as type A (abnormal phenotype, including small, tiny, fused and misplaced otoliths) or type B (abnormal number of otoliths, including increased and decreased otoliths). (B) Morphology of 72hpf WT embryos injected with different morpholinos. One or two cell embryos were injected with 4 ng of S2-SP1 or Mcon. WT. Note the change in otolith size. (C) Schematic representation of two functional domains of zebrafish and human slc26a2 proteins. Degree of identity/similarity indicated for the sulphate transporter, STAS, and the C terminal dimerization domains. (D) Relative numbers of embryos in each category. Single-celled embryos were injected with indicated morpholinos at the indicated dose and categorized at 72hpf. Single-celled S2-SP1 embryos were either uninjected or were injected with 150 pg of human SLC26A2 mRNA and categorized at 72 hpf. (N = number of observed embryos). PHENOTYPE:
|
|
Knockdown of slc26a2 induces semicircular canal defects in larval zebrafish. (A) Changes in semicircular canal morphology and cilia size (scale bars = 30 µm). (B) Changes of neuromasts numbers and hair cell numbers in lateral line (scale bars = 20 µm). (C, D) Changes of cilia numbers in the inner ear (scale bars = 20 µm). (D) Statistical analysis of the ciliary bundles in the inner ear in different types of embryos at 120hpf, including wild type(WT), mismatch-MO control, S2-SP1 MO and Rescue. (E) Statistical analysis of the L1-8 neuromasts numbers in the posterior lateral line in different types of embryos at 120hpf, including WT, Mis-con, S2-SP1 and Rescue. (F) Statistical analysis of the hair cells numbers per neuromast in different types of embryos at 120hpf, including WT, Miscon, S2-SP1 and Rescue. ***P<0.001. PHENOTYPE:
|
|
Knockdown of slc26a2 induces apoptotic signals. Morphology of 120hpf WT AB line zebrafish embryos injected with morpholinos. One- or two-celled embryos were injected with 4 ng of S2-SP1 and Mcon MO (control). WT was injected with RNase-free water. Neuromast hair cells were measured with TUNEL and changes in apoptotic signals in the anterior and posterior lateral neuromasts occurred (scale bars = 10 µm). (B) Statistical analysis of the apoptotic hair cells in anterior and posterior lateral neuromasts in different types of embryos at 120hpf, including WT, S2-SP1 and Rescue, in order to exclude the possibility that the apoptosis hair cells of MO knockdown zebrafish were due to off-target MO toxicity, we co-injected S2-SP1 with p53-MO. ***P<0.001. PHENOTYPE:
|
|
Numbers of supporting cells are unaffected in morphants, while numbers of hair cells decrease in morphants. Morphology of 120hpf Tg(Brn3c:mGFP) S356T transgenic zebrafish expressing GFP in hair cells under control of the POU4F3 promoter embryos injected with morpholinos. One- or two-celled embryos were injected with 4 ng of S2-SP1 and Mcon MO (control). WT was injected with RNase-free water. PHENOTYPE:
|
|
Hearing disability in S2-SP1 zebrafish at 120hpf. M-cells was recorded under sound stimulation (500 Hz, 10 ms). (A) Examples of the recorded EPSC in wild-type (WT), S2-SP1 konckdown, Mcon and rescued individuals. The sound stimuli (80 dB) lasted 10 ms, during which hair cell signals could be induced several times so that a single M-cell could receive input from many different hair cells through ganglion cells because multiple action potentials in the ganglion cells could be produced., As a result, multiple peaks were produced and the EPSC amplitudes were shown for different sound levels. (B) Average C-startle response probability. For each group, 50 larvae were tested. Sound intensities are designated with + and ++ because sound was applied using different units in the M-cell recording and startle response experiments. ***P<0.001. (C) Examples of C-shaped startle responses of different larvae types in response to 500 Hz frequency, 10 ms duration. WT and Mcon-MO larvae usually swam or rested with their backs facing upward, and they tended to swim at one depth. The typical escape response of WT larva was initiated 5–11 ms after sound stimulation, However, S2-SP1 larvae usually remained stationary and rested in abnormal positions. They swam up and down or in circles. Abnormal swimming behaviour of S2-SP1 knockdown zebrafish indicated defective balance. Some S2-SP1 larvae had no response to outside sound stimulation. After rescue, S2-SP1 zebrafish regained their hearing. PHENOTYPE:
|
|
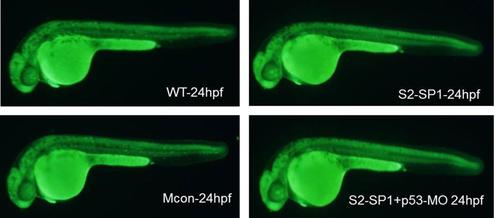
AO straining in wildtype zebrafish, S2-SP1 morphant, S2-SP1 co-injected with p53- morpholino zebrafish. AO straining is shown in S1 Fig. No obvious apoptotic cells were observed among WT, Mcon, and S2-SP1 knock-down zebrafish. There were no differences between S2-SP1 and p53 treated S2-SP1. |