- Title
-
In vivo characterization of human myofibrillar myopathy genes in zebrafish
- Authors
- Bührdel, J.B., Hirth, S., Keßler, M., Westphal, S., Forster, M., Manta, L., Wiche, G., Schoser, B., Schessl, J., Schröder, R., Clemen, C.S., Eichinger, L., Fürst, D.O., van der Ven, P.F., Rottbauer, W., Just, S.
- Source
- Full text @ Biochem. Biophys. Res. Commun.
|
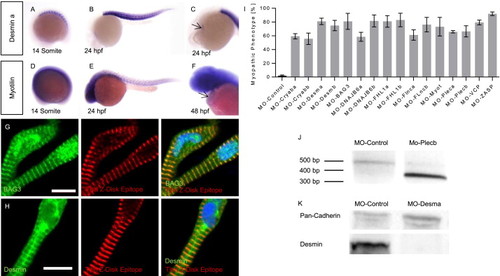
MFM disease gene expression analysis and knock-down in zebrafish (A–E) Whole-mount antisense RNA in situ hybridization shows desmin-a and myotilin expression in skeletal muscle fibers at the 14 somite stage and 24 hpf. (C,F) At 24/48 hpf cardiac expression of desmin a/myotilin was observed (arrows). (G) As revealed by co-stainings with an antibody against Z-disk Titin (T12) or DAPI, respectively, BAG3 localizes to sarcomeric Z-disks and nuclei of zebrafish cardiomyocytes. (H) Desmin is localized at the sarcomeric Z-disk, scale bars = 10 µm. (I) Percentage of injected embryos showing a myopathic phenotype. (J) Injection of MO-Plecb blocks the splice donor site of exon 7 of Plectin b, leading to an abnormally short cDNA fragment due to skipping of exon 7. (K) Western-blot analysis shows the reduction of Desmin after injection of MO-Desma. Pan-Cadherin is used as loading control. EXPRESSION / LABELING:
|
|
Loss of MFM gene function results in a progressive motility deficiency (A–C) Spontaneous movement assay with false-colored superimposed overviews of 24 hpf control (A) and MFM morphants (B–C); Red pictures = 0 s; green pictures = 10 s. (D) Summary of voluntary movement at 24 hpf of MFM morphants. (E) Quantification of flight reaction to a touch stimulus of 72 hpf individuals. Darker gray bars indicate the effect of Morpholinos directed against non-MFM genes as controls. Asterisks indicate significance compared to the MO-control group. PHENOTYPE:
|
|
Disturbed myofibrillar structure in MFM gene deficient embryos (A–C) Lateral views of a control zebrafish (A) at 72 hpf showing bright homogenous birefringence compared to reduced birefringence in Desmin-a (B) and DNAJB6-b (C) morphants. (D) Quantification of birefringence in Desmin-a and DNAJB6-b morphants. (E–L) Electron microscopy of MFM gene deficient and control embryos at 72 hpf. (E) Nuclei (dotted arrows) in the skeletal muscle are elongated in controls; (F,H) rounded nuclei in myopathic muscle. (I) Control injected embryos develop highly ordered myofibrils with ordered sarcomeric units, Z-disks, M-Bands and A/I regions. (F,H) Vacuolization in MO-Desma and MO-crayaba injected embryos (asterisk). (G) Autophagic pathology in Dnajb6-a morphants. (F,J,K) Mitochondrial alteration in Desmin-a, Plectin-b and αB-crystallin-a morphants (arrowheads). (L) Stress fiber-like structures in Myotilin morphants (arrows). Scale bars 1 µm. PHENOTYPE:
|
|
Loss of MFM gene function results in a cardiomyopathy in zebrafish (A–D) Phenotype of MO-control (A), MO-Cryaba (B), MO-Cryabb (C) and MO-Myot (D) zebrafish hearts at 72 hpf. (E,F) Heart rates of MFM morphants at 48 (E) and 72 hpf (F) are significantly reduced. (G,H) Fractional shortening at defined developmental stages (48, 72, 96 hpf) of atrial (G) and ventricular (H) chambers. |
|
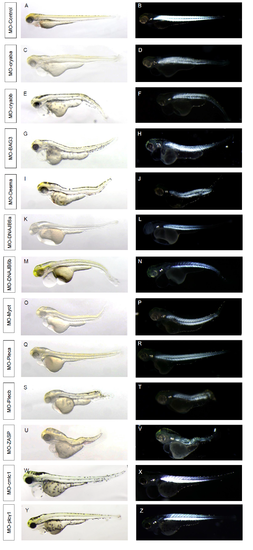
Morphology of MFM gene morphants and birefringence imaging. PHENOTYPE:
|