- Title
-
Regulation of gene expression mediating indeterminate muscle growth in teleosts
- Authors
- Ahammad, A.K., Asaduzzaman, M., Asakawa, S., Watabe, S., Kinoshita, S.
- Source
- Full text @ Mech. Dev.
|
EGFP expression in zebrafish larvae injected with MYHM2528-1 reporter constructs. A-R, EGFP expression in myotomal skeletal muscle of zebrafish embryos injected with reporter constructs, P5000 (A,B), P4000 (C,D), P3000 (E,F), P2500 (G,H), P2300 (I,J), P2100 (K,L), P1500 (M,N), P1000 (O,P) and P600 (Q,R). Head to left in all panels. Right side panels are the magnified view of boxed areas of the left side panels. Scale bars: 100 µm. |
|
Expression patterns of EGFP in theTg:MYHM2528-1:EGFP stable transgenic line embryo and larva. A stable line was established by injecting the P2100 construct. EGFP expression was observed in the whole myotomal region at 1dpf (A,B), 2dpf (C), and 3dpf (D). In addition to myotomal skeletal muscle, the craniofacial muscle also expresses EGFP at 3dpf (E). Scale bars: 100 µm. |
|
Localization of EGFP-expressing myotomal muscle fibers in Tg:MYHM2528-1:EGFP larvae. (A–F) Transverse section of myotomal region of Tg:MYHM2528-1:EGFP at 3 dpf. (A–C) Slow muscle fibers were stained red with a F59 antibody (A, F59 antibody; B, EGFP; C, merged view). (D–F) The magnified view of boxed areas of panels A–C. (G–L) Fast muscle fibers were identified with a F310 antibody (G, F310 antibody; H, EGFP; I, merged view). The dotted line in panel I indicates the middle of the myotomal compartment. EGFP-positive fast muscle fibers are predominantly distributed in the outer region. (J–L) The magnified view of boxed areas of panels G–I. EGFP-expressing slow muscle fibers are indicated by arrowheads in panels A–F, H, I, K, L. Scale bars: 50 µm. |
|
Localization of EGFP-expressing myotomal muscle fibers in Tg:MYHM2528-1:EGFP at early juvenile (20 dpf, 8 mm standard length (SL), late juvenile (40 dpf, 17 mm SL) and adult (60 dpf, 25 mm SL) stages). (A) Schematic of a cross-section of the myotomal region. Slow muscle is distributed throughout the base of the dorsal fin (erector and depressor (ED) slow) and lateral surface (lateral slow). (B) Body size-related increase in muscle fiber numbers in slow and fast muscles of zebrafish. Data cited from Lee (2010). (C–H) Cross-sectional view of the myotomal region at the early juvenile stage. Muscle fibers were stained with MF20 (C,E,F,G) and BIODIPY TR Ceramide (D,H). EGFP expression was observed in small diameter fast muscle fibers (C,D,G,H), the inner part (near the septum between slow and fast muscles) of lateral slow (E) and ED slow (F) muscles. In panel C and G, an EGFP-positive small ‘neonatal’ muscle fiber and the surrounding large ‘old’ fibers are marked by an arrowhead and asterisks, respectively. (I–N) Cross-sectional view of the myotomal region at the late juvenile stage. Muscle fibers were stained with MF20 (I,K,L,N) and BIODIPY TR Ceramide (J,M). EGFP expression was not observed in fast muscle (I,G,L,M). In the inner portions of lateral slow (K) and ED slow (N) muscle fibers expressed EGFP at the early juvenile stage. (O–Q) Cross-sectional view of the myotomal region at the adult stage. Muscle fibers were stained with MF20. In consist with late juvenile stage, EGFP expression was not observed in fast muscle (O) but expressed at the inner portions of ED slow (P) and lateral slow (Q) and muscle fibers. Arrow heads indicate positive EGFP expression. Scale bar: 50 µm. |
|
Adaxial cell-derived slow muscle progenitor in Tg:MYHM2528-1:EGFP transgenic embryo. (A) Ten somite stages of Tg:MYHM2528-1:EGFP transgenic embryo by fluorescent microscope observation. Lateral view. No EGFP expression was observed. (B–D) Immunohistochemistry of Tg:MYHM2528-1:EGFP transgenic embryo at 10 somite stage. Dorsal view. (B) F59 antibody; (C) EGFP; and (D) merged view. Scale bars: 50 µm. In panel B, adaxial cell-derived slow muscle progenitors are indicated by arrowheads. |
|
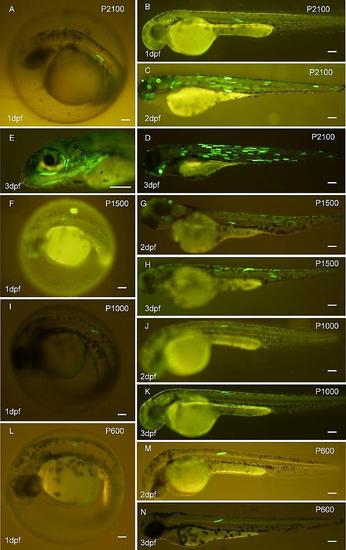
EGFP expression patterns in the myotomal region of the zebrafish embryo (1 dpf, A, B) and larva (2 dpf, C; 3dpf, D) injected with the P2100 reporter construct. (D, E) EGFP expression patterns in craniofacial muscles in zebrafish larvae at 3 dpf. As well, zebrafish embryo (1 dpf, F), (1 dpf, I), (1 dpf, L) and larva (2dpf,G; 3dpf,H), (2dpf, J; 3dpf,K), (2dpf,M; 3dpf,N) injected with P1500, P1000, P600 reporter constructs, respectively. Scale bars: 100 µm. |
|
Immunohistochemistry localizing EGFP expression in both fast and slow muscle fibers of P2100-injected zebrafish larvae. (A–C) Slow muscle fibers expressing EGFP as reacted with F59 antibody (A, F59 antibody view; B, F59 with EGFP; and C, F59 and EGFP with DAPI) in a P2100-injected larva at 3 dpf. (D–F) Fast muscle fibers expressing EGFP as reacted with F310 antibody in a P2100-injected larva at 3 dpf (D, F310 antibody stained view; E, F310 with EGFP; F, F310 and EGFP with DAPI). Scale bars: 50 µm. |
|
Influence of Hedgehog (Hh) signaling pathway on the MYHM2528-1 promoter. Hh signaling was inhibited by cyclopamine treatment. Fertilized eggs of Tg:MYHM2528-1:EGFP were transferred into 2–10 µg mL1 cyclopamine solution. Control embryos were developed without cyclopamine. (A–F) Morphology and EGFP expression of control (A–C) and 10 µg mL1 cyclopamine-treated (D–F) transgenic line. (A,D) Ventral views of head of zebrafish larvae at 3 dpf. Inhibition of Hh signaling resulted in a fused-eye phenotype (D). EGFP expression in larvae at 2 dpf (B,E) and 3 dpf (C,F). Cyclopamine-treated larvae displayed a curled body phenotype resulting from Hh signal inhibition but maintained normal EGFP expression in myotomal muscle fibers. (G) The rate of zebrafish embryos expressing EGFP in the larvae at 3 dpf with and without cyclopamine treatment. (H–K) Transverse section of myotomal region of 10 µg mL1 cyclopamine-treated larvae of Tg:MYHM2528-1:EGFP at 3 dpf. (H) Primary slow muscle fibers disappeared and secondary slow muscle fiber was stained with F59 antibody. I, merged view showing reacted with F59 and EGFP antibodies. EGFP expressing slow muscle fibers are located at the dorsal extreme region. (J,K) The magnified view of boxed areas of panels H and I. Arrowheads in panels H–K indicate EGFP expressing slow muscle fibers. Scale bars: 100 µm. |
Reprinted from Mechanisms of Development, 137, Ahammad, A.K., Asaduzzaman, M., Asakawa, S., Watabe, S., Kinoshita, S., Regulation of gene expression mediating indeterminate muscle growth in teleosts, 53-65, Copyright (2015) with permission from Elsevier. Full text @ Mech. Dev.