- Title
-
Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL
- Authors
- Burgoyne, T., O'Connor, M.N., Seabra, M.C., Cutler, D.F., Futter, C.E.
- Source
- Full text @ J. Cell Sci.
|
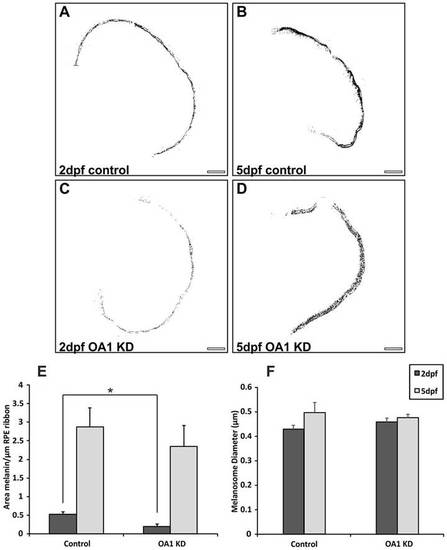
OA1 MOs reduce melanosome number at 2dpf without affecting melanosome size. (A–D) Contrast-enhanced images of zebrafish eye cross-sections highlighting only electron-dense melanin. Images are shown from (A,B) control and (C,D) OA1 MO-treated zebrafish. KD, knockdown. Scale bars: 20µm. (E) At 2dpf there is a significant reduction in melanin area between controls and OA1 MO-treated zebrafish. By 5dpf, the OA1 MO appears to be less effective, resulting in no significant difference in melanin area between controls and OA1 MO-treated animals. (F) The OA1 MO had no apparent effect on melanosome diameter at 2 and 5dpf. Results show the mean±s.e.m.; *P<0.05 (Student′s t-test). |
|
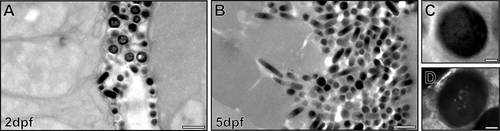
Intense biogenesis of mature melanosomes between 2 and 5dpf. Electron micrographs of zebrafish RPE show a large increase in melanosome number between 2dpf (A) and 5dpf (B). (C,D) High-magnification images of mature melanosomes at 2dpf. Some mature melanosomes have an appearance of ‘holes’ (D). Scale bars: 1µm (A,B), 100nm (C,D). |
|
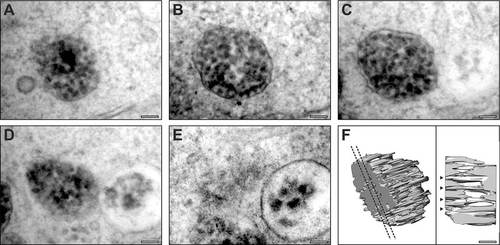
Serial section electron microscopy analysis of tyrosinase MO-treated RPE reveals immature melanosomes that contain fibrils. (A–E) Serial section micrographs of the same immature melanosome in an RPE cell from a tyrosinase MO-treated zebrafish at 5dpf. (F) 3D rendering of the micrograph data reveals fibrils running through the melanosome. Dotted lines indicate the position of the slice shown in the right-hand panel; arrowheads indicate fibrils running through the entire thickness of the melanosome. Scale bars: 100 nm. PHENOTYPE:
|
|
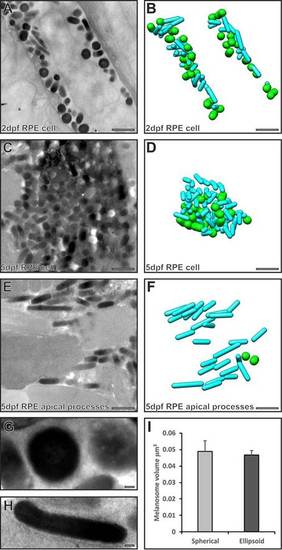
Serial section electron microscopy analysis reveals two populations of melanosomes in the RPE cell body, but only cylindrical ones enter the apical processes. (A,C,E) Single electron micrographs from a stack of serial section images used to generate models (B,D,F) of spherical (green) and cylindrical (cyan) melanosomes. (A,B) Both cylindrical and spherical melanosomes are found in the RPE cell body at 2dpf. (C,D) Cylindrical and spherical melanosomes are densely packed in the RPE cell body at 5dpf. (E,F) Only cylindrical melanosomes are in the apical processes at 5dpf. (G,H) Higher-magnification images of a spherical melanosome (G) and a cylindrical melanosome (H). Scale bars: 1µm (A–F), 100 nm (G–H). (I) Spherical and cylindrical melanosomes have similar volumes. Results show the mean±s.e.m. |
|
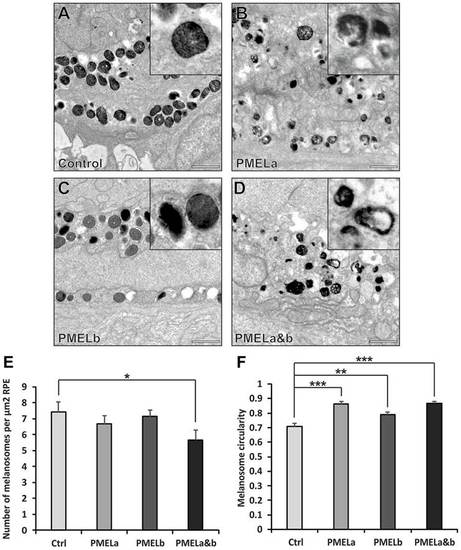
PMELa MO reduces the number of cylindrical melanosomes to a greater extent than PMELb MO. (A–D) Electron micrographs highlighting melanosome morphology at 2dpf for zebrafish injected with control (A), PMELa (B), PMELb (C) and PMELa&b MOs (D). Scale bars: 1µm. (E) The PMELa&b MO resulted in a decrease in the number of melanosomes. Ctrl, control. (F) All PMEL MOs resulted in a significant loss of cylindrical melanosomes. PMELa had a greater effect on melanosome shape than the PMELb MO. Results show the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 (Student′s t-test). PHENOTYPE:
|
|
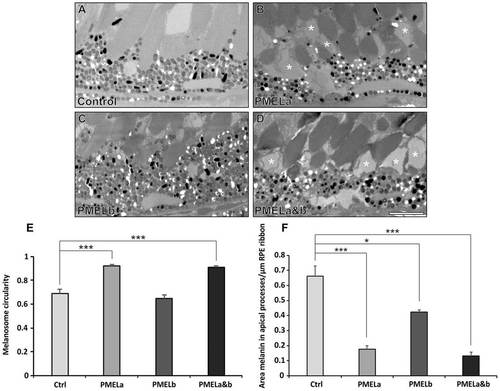
PMELa MO prevents melanosome movement into the apical processes and has a profound effect on photoreceptor morphology. (A–D) Electron micrographs showing the effect on melanosome positioning in the apical processes at 5dpf for zebrafish injected with control (A), PMELa (B), PMELb (C) and PMELa&b MOs (D). (B,D) PMELa and PMELa&b MOs resulted in missing or disrupted outer segments as shown by the asterisks. Scale bars: 4µm. (E) Photoreceptors in fish injected with PMELa and PMELa&b MOs had significantly fewer cylindrical melanosomes at 5dpf. Ctrl, control. (F) PMELa and PMELa&b MOs resulted in a reduced area of melanin (thus fewer melanosomes) in the apical processes. Results show the mean±s.e.m.; *P<0.05, ***P<0.001 (Student′s t-test). PHENOTYPE:
|