- Title
-
Nodal Signaling Range Is Regulated by Proprotein Convertase-Mediated Maturation
- Authors
- Tessadori, F., Noël, E.S., Rens, E.G., Magliozzi, R., Evers-van Gogh, I.J., Guardavaccaro, D., Merks, R.M., Bakkers, J.
- Source
- Full text @ Dev. Cell
|
Zygotic and Maternal Zygotic aoh Mutants Display Laterality Phenotypes and Defects in the Nodal Signaling Pathway (A) Lateral view of WT and Zaoh mutant embryos at 48 hpf. (B) Characterization of the Zaoh mutant heart and gut phenotype. ISH at 28 hpf for myl7 and at 55 hpf for Foxa3 (dorsal view; anterior top). (C) At 26 hpf, MZaoh mutant embryos displayed slight developmental delay and a mild dorsalization phenotype in the tailfin (arrowhead). At 55 hpf, incidence of reversed gut looping and organ position was observed (arrowhead pointing at liver). (D) ISH for spaw (arrowhead indicates anterior-ward expansion) in WT, Zaoh, and MZaoh embryos. (E) FurinA protein and functional domains. Star, aoh mutation. (F) Agarose beads preincubated in recombinant BMP and Nodal protein were implanted in MZaoh mutant embryos at 15 somites. Analysis of heart position was determined by myl7 ISH. (G and H) Spaw-EGFP fusion protein (G). Furin recognition sites 1 and 2 are underlined. Changes in amino acids in the noncleavable forms are detailed (H) western blot with anti-GFP. Incubation of Spaw with Furin results in cleavage and appearance of the mature 41 kDa Spaw-EGFP (asterisk). Incubation of the different Spaw-EGFP variants with Furin demonstrates that cleavage happens only at the single (optimal) predicted Furin cleavage site 1. (I) Dorsalization phenotypes induced by injection of Spaw-EGFP variants mRNA (1 picogram [pg]) at 24 hpf. Abbreviations, liver (l), pancreas (p), and gut (g). EXPRESSION / LABELING:
|
|
FurinA Is Required Cell-Autonomously to Process Spaw and to Confer Its Long-Range Signaling Activity (A) Schematic of the transplantation procedure. (B and C) Both MZspaw-/- (B) and MZaoh-/- (C) embryos fail to express spaw in the left LPM at 18 somites. (D) ISH for spaw (blue staining) with immunolabeling for transplanted cells (red staining). Transplantation of spaw-injected spaw-/- cells (yellow clone, red outline) in MZspaw-/- resulted in the rescue of spaw expression in the left LPM several cell diameters away from the transplanted clone. (E) Transplantation of cells injected with RNA of a noncleavable form of spaw (yellow clone, purple outline) in MZspaw-/- failed to induce of spaw expression in the left LPM. (F) Transplantation of spaw-injected spaw-/- cells (yellow clone, red outline) in MZaoh-/- resulted in induction of spaw expression in the left LPM several cell diameters away from the transplanted clone. (G) Spaw-injected aoh-/- cells (green clone, red outline) in MZspaw-/- were unable to induce spaw expression in the left LPM. In all schematics, MZspaw-/- cells appear in yellow and MZaoh-/- cells in green. EXPRESSION / LABELING:
|
|
FurinA-Mediated Processing of Spaw Specifically Correlates with Extracellular Localization of Spaw (A) Embryos were injected at the one-cell stage with 50 pg of Spaw-EGFP mRNA or Spaw-Δ1 mRNA together with a membrane-bound tdTomato fusion protein and imaged at sphere stage (4 hpf). Addition of FurinA mRNA (30 pg) to the injection mix resulted in an increased extracellular localization of Spaw-EGFP, but not Spaw-Δ1. (B) Schematic representation of the Sqt-EGFP fusion protein (Müller et al., 2012). The Furin recognition site is underlined in the detail of the sequence. The changes in amino acids in the noncleavable form Sqt-Δ are detailed. (C) Removal of the Furin cleavage site (Sqt-Δ) resulted in reduced extracellular localization of Sqt-EGFP. mRNA injections and imaging were carried out identically to (A). (D) MZaoh mutant embryos were injected at the one-cell stage with 50 pg Sqt-EGFP mRNA and imaged at sphere stage (4 hpf). Arrows indicate extracellular localization of the Spaw-EGFP and Sqt-EGFP fusion proteins respectively in (A), (C), and (D). (E) Quantification of the dorsalization phenotypes induced by injection of 1 pg Sqt-EGFP and Sqt-Δ. The embryos were scored as described in Figure 1I. |
|
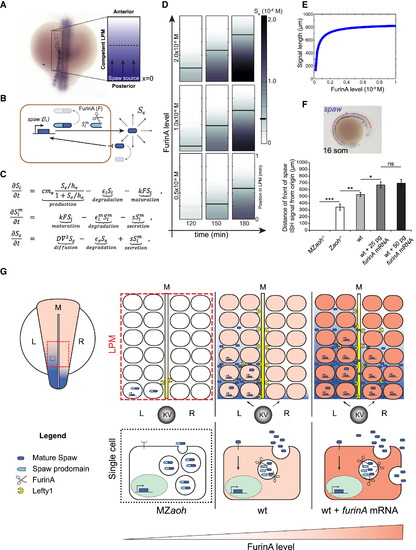
FurinA Levels Control the Expansion of the Spaw Expression Domain in the LPM (A and B) For the purpose of mathematical modeling, we have considered the left LPM of the developing zebrafish embryo as a linear domain (displayed as a rectangle here) with a source of Spaw at the posterior end (x = 0); (B) behavior of Spaw in the competent LPM described in (A). Synthetized intracellular Spaw (Si), mature intracellular Spaw (Sim), and extracellular Spaw (Se). (C) Partial-differential equation model of the system described in (B). Parameter definitions, analysis of the model, and details on the numerical simulation and boundary conditions are available in Supplemental Experimental Procedures. (D) Snapshots of Movie S1 showing a simulation of the model defined in (C). The speed of progression of Se, and consequently of the domain of spaw expression in the LPM, increases with the level of FurinA. (E) The model predicted that increasing FurinA levels, resulting in enhanced maturation of Spaw, results in increased length of the spaw expression domain at a given time (180 min here). (F) Quantification of the length of the spaw expression domain (anterior-posterior) in embryos with no (MZaoh; n = 12), low (Zaoh mutants; n = 7), normal (WT; n = 15), or high (WT injected respectively with 25 pg; n = 25 and 50 pg; n = 17 furina mRNA) FurinA levels. Histograms display average value ± SEM; p < 0.05, p < 0.01, and p < 0.005 in Student’s t test. (G) Cartoon illustrating the effect of FurinA on the signaling range of Spaw in the LPM. In a WT situation, Spaw is cleaved prior to secretion by cells at the posterior end of the LPM (10 somite stage, 13 hpf). Spaw induces its own expression in a paracrine fashion, and the spaw expression domain expands toward the anterior end of the developing LPM, reaching the heart field at the 23-somite stage (20 hpf). Spaw also induces expression of Lft1 at the midline, which prevents it from reaching the right LPM. Spaw expression is consequently limited to the left LPM and establishes LR patterning. In the MZaoh mutants, the absence of FurinA processing of Spaw results in failure to induce Spaw expression in the LPM and of Lft1 in the midline. As a consequence, LR patterning is affected. Overexpression of FurinA results in increased presence of mature Spaw in the extracellular space. The activation of Spaw in the LPM progresses faster toward the anterior left LPM. LR patterning is affected, likely as a result of an excess of Spaw protein overcoming the Lft1 midline barrier, Kupffer’s Vesicle (KV) and midline (M). |
|
Aoh is a furina mutant. Laterality phenotypes of Zaoh and MZaoh. (A) Anterior and posterior expression of laterality markers in wt, Zaoh and MZaoh embryos. Reduced but maintained expression of pitx2c in Zaoh is indicated by arrowhead. (B) Genomic position of the aoh mutation. (C) A single nucleotide substitution in furinA (G to A) resulting in a tryptophan (Trp) to stop (TAG) mutation at amino acid 411 is responsible for the aoh mutation. (D) Complementation test. Outcross of aoh+/- to stu+/- (Walker et al., 2006) fails to complement the aoh cardiac jogging phenotype. Stacked histograms display averages +/- SEM collected over 3 independent biological replicates. (E) FurinA is maternally contributed to the embryo and is broadly expressed during somatogenesis in wt and sfw/spaw-/- embryos. (F) Addition of FurinA mRNA to Spaw-eGFP mRNA injected in 1-cell embryos results in significantly reduced Spaw-eGFP precursor (*) in 12-somite stage embryos. Whole embryo extracts were subjected to immunoblotting with the indicated antibodies. Actin is shown as loading control. Mature Spaw-eGFP (**) appears as a faint band of approximately 41 kDa. Quantification of the SpaweGFP precursor abundance is depicted in the histogram. *** P<0.001 (one-way ANOVA). Data collected over three biologically and technically independent replicates. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Long-range signalling activity of recombinant mature Nodal. (A) Agarose beads incubated in recombinant mature mouse Nodal protein implanted in sfw/spaw mutant embryos at 13 somite stage induced spaw expression at long range. |
|
Localization and long-range signalling activity of Spaw in the presence of FurinA. (A) Extracellular mature Spaw-eGFP can be observed in the LPM of wt embryos (upper row, white arrows). In Zaoh-/-, no extracellular Spaw-eGFP fluorescence can be detected. (B) Schematic of the transplantation procedure. Donor embryos were injected at 1-cell stage with mRNA of a spaw variant and furina depending on the experiment. At sphere stage, approximately 50 cells were isolated from the donor embryos and transplanted into the animal pole of sphere stage recipient embryos, which were subsequently left to grow for approximately 75 min. (C) After fixation ntla expression was determined by in situ hybridisation (purple staining) and the localisation of the transplanted cells by immunolabelling (brown staining). Expression pattern classes resulting from increasing ntla induction were defined similar to described in (Muller et al., 2012) and used for quantification of the results shown in (D). |
|
Overexpression of FurinA perturbs the laterality of Spaw expression. Wt and Zaoh mutant embryos injected at the 1-cell stage with furinA mRNA were assayed for spaw expression by ISH at 16 somites (17 hpf) and 20 somites (19 hpf). The observed expression patterns were separated in classes presented in (A) and quantified for each condition in (B) and (C). At 16 somites (B), the expected pattern of expression is “middle” in normally developing wt embryos, while it is mainly “low” in Zaoh. Additionally to the increased expansion of the spaw expression domain, note that overexpression of furinA results in the appearance of bilateral and right-sided expression of spaw. (C) At 20 somites, the expected pattern of expression is “high” in wt embryos. |
Reprinted from Developmental Cell, 32(5), Tessadori, F., Noël, E.S., Rens, E.G., Magliozzi, R., Evers-van Gogh, I.J., Guardavaccaro, D., Merks, R.M., Bakkers, J., Nodal Signaling Range Is Regulated by Proprotein Convertase-Mediated Maturation, 631-9, Copyright (2015) with permission from Elsevier. Full text @ Dev. Cell