- Title
-
CNS Myelination Requires Cytoplasmic Dynein Function
- Authors
- Yang, M.L., Shin, J., Kearns, C.A., Langworthy, M.M., Snell, H., Walker, M.B., Appel, B.
- Source
- Full text @ Dev. Dyn.
|
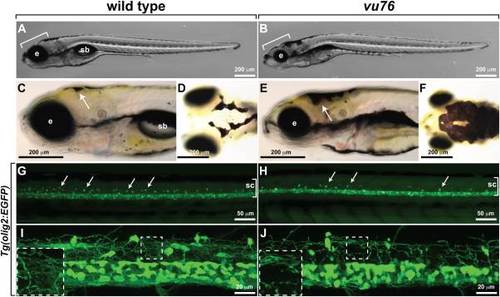
The vu76 mutation causes defects in pigment deposition and oligodendrocyte development. Images of living wild-type (A) and vu76 mutant (B) larvae at 6 dpf. The mutant has a smaller brain (bracket) and eye (e) than wild-type and lacks a swim bladder (sb). Lateral (C,E) and dorsal (D,F) views of 5 dpf wild-type and vu76 mutant larvae to show pigment patterns. The dark pigment of the mutant is more broadly distributed than that of the wild-type. Low (G,H) and high (I,J) magnification images of Tg(olig2:EGFP) reporter gene expression in wild-type and mutant larvae. Images are focused on the trunk spinal cord (sc, brackets) with dorsal up. In G and H dorsally migrated OPCs appear as green dots (arrows). Fewer dorsal OPCs are apparent in the vu76 mutant larva than in the wild-type larva. At 6 dpf OPC and oligodendrocyte processes form a dense meshwork (I, boxed area magnified as inset). Oligodendrocyte lineage cell processes are evident in the mutant larva, but the density of processes is less than in wild-type (J, boxed area magnified as inset). |
|
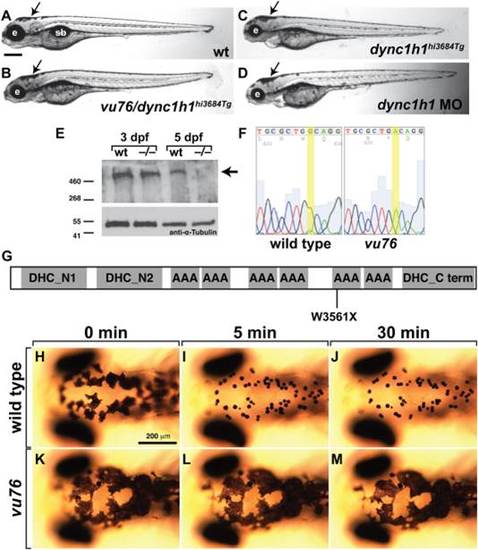
The vu76 allele is a mutation of dync1h1. A?D: Compound heterozygous vu76/dync1h1hi3684Tg, homozygous dync1h1hi3684Tg and dync1h1 MO-injected larvae have similar phenotypic characteristics including small heads and small eyes (e) and abnormally dispersed pigment (arrows). E: Western blotting of protein extracts obtained from 3 and 5 dpf larvae with anti-Dync1h1 antibody reveals an approximately 500 kD protein present in 3 and 5 dpf wild-type larvae and 3 dpf mutant larvae but absent from 5 dpf mutant larvae (arrow). The blot was probed with anti-α-Tubulin antibody as a loading control. F: Sequencing of dync1h1 cDNA from wild-type and vu76 mutant larvae revealed a G to A transition changing a tryptophan codon to a stop codon. This change is predicted to prematurely terminate translation at amino acid position 3561, within the motor domain of Dync1h1 (G). H?M: Whereas 3 dpf wild-type larvae rapidly aggregate melanosomes into tight spots upon treatment with epinephrine, which reflects retrograde transport of melanosomes on microtubules, melanosome distribution in vu76 mutant larvae is not changed by epinephrine. Images in H?M show dorsal views of the head. Elapsed time following initiation of epinephrine treatment is indicated above the panels. PHENOTYPE:
|
|
Dync1h1 deficient zebrafish have a deficit of oligodendrocytes, each of which has fewer myelin sheaths. A,B: Immunohistochemistry to detect Sox10 (red) in combination with olig2:EGFP (green) marks oligodendrocyte lineage cells in 4 dpf wild-type and dync1h1vu76 mutant larvae. Panels show representative images of transverse sections through the trunk spinal cord, with dorsal up. Anti-acetylated Tubulin staining (blue) marks axons. C: Mutant larvae have approximately 50% the normal number of oligodendrocyte lineage cells. D,E: Data, collected from time-lapse movies, showing fewer dorsally migrating OPCs and fewer OPC divisions in dync1h1 MO-injected larvae than in wild-type. F,G: Representative images of single, sox10:EGFP-CaaX+ oligodendrocytes in 6 dpf control (F) and dync1h1vu76 mutant (G) larvae. Asterisks mark cell bodies and arrows mark ends of single myelin sheaths. H: Quantification revealed fewer sheaths in dync1h1vu76 mutant larvae than in control. I: Graph showing that average myelin sheath length is slightly longer in dync1h1vu76 mutant larvae than in wild-type. However, the statistical significance of the difference is weak (P = 0.0414). J: Box-and-whisker plot revealed that both the minimum (min) and maximum (max) lengths of sheaths in dync1h1vu76 mutant larvae were slightly more variable than in control larvae. However, the differences between the average maximum and minimum lengths were not significant (ns). K: Graph showing the average total myelin sheath lengths of individual oligodendrocytes. Error bars represent ▒ SEM. For panels H?K, wild-type data were collected from 28 cells in 11 larvae. Mutant data were collected from 15 cells in 9 larvae. Statistical significance was determined by Student′s t-test for panels C?E and by nonparametric two tailed Mann-Whitney t-test for panels H?K. ***P < 0.0001; **P < 0.001; *P < 0.05. |
|
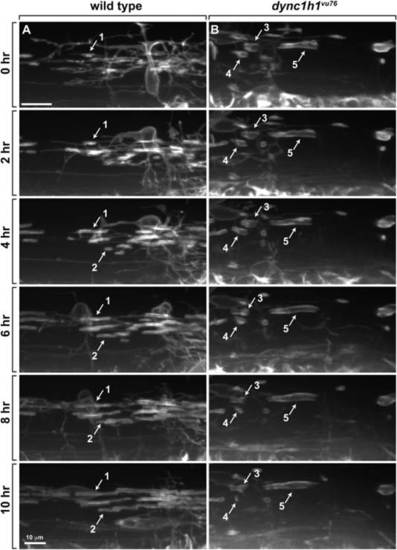
Axon wrapping by oligodendrocyte membrane is abnormal in dync1h1vu76 mutant larvae. Panels show images captured from time-lapse movies of oligodendrocytes marked by Tg(nkx2.2:EGFP-CaaX) reporter expression beginning at 2.5 dpf. Time elapsed since beginning of movies is marked on the left. Images were obtained from lateral view of the trunk spinal cord, with dorsal up. A: In wild-type larvae, oligodendrocyte membrane processes contact axons, initiate tight wrapping and lengthen bi-directionally to form myelin sheaths (see example sheaths 1 and 2). Once formed, most sheaths are stable and rarely lost. B: In dync1h1vu76 mutant larvae, some sheaths do not lengthen (e.g., sheath 3) and some shorten and are subsequently lost (e.g., sheath 4). Sheath 5 forms a relatively long segment. |
|
Normal levels of CNS myelin gene expression require Dync1h1 function. A?F: Whereas 4 dpf wild-type larvae robustly express cldnk, plp1a, and mbp RNA, only apparently low levels of transcripts are evident in similarly processed dync1h1vu76 mutant larvae. Images show dorsal views of whole larvae at the level of the midbrain and hindbrain, with anterior to the left. G,H: Quantitative RT-PCR confirmed the deficit of myelin gene expression in dync1h1vu76 mutant larvae and dync1h1 MO-injected larvae. I,J: Levels of Mbp (red), detected by immunohistochemistry, appear higher in wild-type spinal cord than in mutant spinal cord. Anti-acetylated Tubulin (green) reveals axons. Images show transverse sections, with dorsal up. Statistical analysis was performed using an unpaired t test with Welsh correction. *P < 0.05; **P < 0.001. Error bars represent + SEM. EXPRESSION / LABELING:
|
|
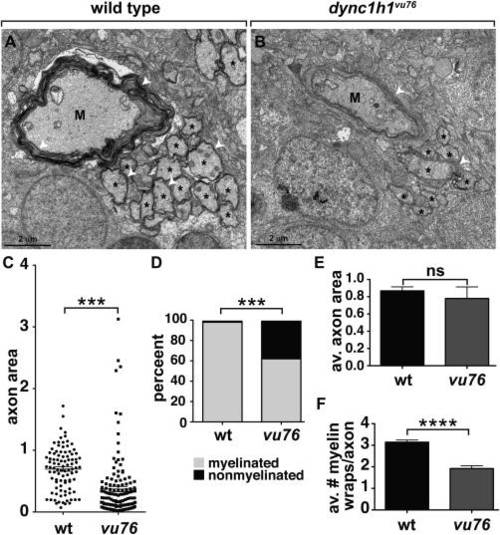
Loss of Dync1h1 function causes CNS axon and myelin abnormalities. A,B: EM micrographs of ventral spinal cords of 6 dpf wild-type and dync1h1vu76 mutant larvae. M indicates Mauthner axon, asterisks indicate intermediate sized axons and white arrowheads mark myelin membrane. C: Scatter plot showing cross sectional area of axons. Mauthner axons were excluded from the plot. The average area of axons in mutant larvae is reduced relative to wild-type. D: Graph showing percentage of myelinated, intermediate size axons at 6 dpf. Intermediate was defined as axons having a cross sectional area between 0.201 and 3.99 Ám. Loss of Dync1h1 function results in more nonmyelinated axons. E: Graph showing the average axon cross sectional area of intermediate class myelinated axons. F: Graph showing the average number of myelin wraps on intermediate class myelinated axons. Statistical analysis for the proportion of myelinated and nonmyelinated axons was performed using a two-sided Chi-square test. Statistical analysis for axon area and myelin wraps was performed using two-tailed, unpaired t-test. ***P < 0.0005, ****P < 0.0001. ns, not significant. Error bars represent + SEM. PHENOTYPE:
|