- Title
-
Axon-Schwann cell interactions during peripheral nerve regeneration in zebrafish larvae
- Authors
- Ceci, M.L., Mardones-Krsulovic, C., Sánchez, M., Valdivia, L.E., Allende, M.L.
- Source
- Full text @ Neural Dev.
|
Neuromast reinnervation after PLL nerve regeneration. Tg(neurod:TagRFP) fish that have a red labeled pLL nerve were injected at the one cell stage with the pE46:GFP DNA construct and were selected if they displayed green fluorescence in a single sensory neuron in the pLL ganglion (inset in A). The innervation pattern of the single sensory neuron was recorded both before neurectomy and after regeneration of the axon. Two different examples of reorganization during pLL nerve regeneration are shown, referred to as larva 1 (A-F) and larva 2 (G-J). Larva 1 shows a pLL ganglion neuron that innervates the terminal neuromasts before injury (B, D). Neurectomy is carried out about 200 µm away from the ganglion severing all axons of the pLL nerve (C). Twenty-four hours post neurectomy (hpn), the pLL nerve has regenerated about half way down the body of the larva (E). At 48 hpn, the nerve has completely regenerated (F) altough the green-labeled axon now innervates a different neuromast, the L4 (small inset in F) and does not innervate its original targets, which are innervated by other neurons (large inset in F). Larva 2 shows innervation of the terminal neurmasts before injury (G, H) and, after neurectomy and regeneration, the same neuromasts are reinnervated (I, J). Scale: A, B, E, F, G, I: 200 µm; C: 100 µm; inset in A, D, larger inset in F, inset in G, H; small inset in F: 20 µm. EXPRESSION / LABELING:
|
|
Axonal and Schwann cell behaviors at the point of neurectomy. Three days post fertilization transgenic Tg(neurod:TagRFP;foxd3:GFP) zebrafish larvae were neurectomized and imaged from 5 hpn to 11 hpn every 2 min following complete nerve transection (axons in red and Schwann cells in green). In all panels, the arrows show the behavior of axons and Schwann cells proximal to the gap whereas the arrowhead shows the behavior of Schwann cells distal to the gap. (A, B) A few hours after neurectomy, distal (denervated) Schwann cells extend their processes within the gap and show an exploratory behavior, whereas proximal Schwann cells are less motile. (C) At 6.5 hpn, the regrowing axons have contacted distal Schwann cells and have formed a bridge across the gap (asterisk). (D, E) After 7 hpn, the axons complete the crossing of the gap; often, the first axon to navigate the gap stops growing and another axon takes the lead. (F) After 11 hpn, the regrowing nerve has grown past the gap enabling the reconnection between proximal and distal Schwann cells in 100% of neurectomized larvae. EXPRESSION / LABELING:
|
|
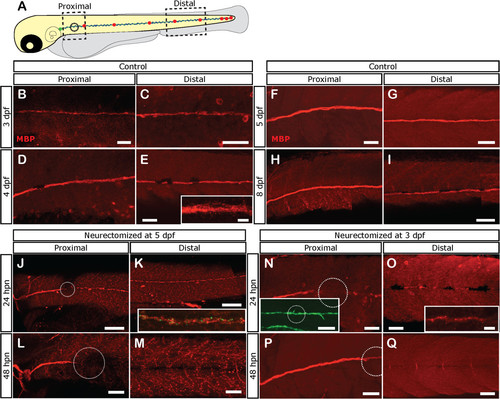
Loss of Schwann cell differentiation markers after denervation. Tg(foxD3:GFP) larvae were left untreated or neurectomized and then fixed at different times and processed for immunostaining with anti-Myelin Basic Protein (MBP; labeled in red). (A) The expression of MBP was evaluated in a proximal and distal area with respect to the point of neurectomy (black circle). (B-I) In control larvae. MBP is expressed at proximal and distal levels at 3 dpf and increases as the larva ages. (J-M) Expression of MBP becomes absent after neurectomy in denervated Schwann cells. Five-day-old tg(NeuroD:GFP) larvae were neurectomized and were fixed and processed for immunostaining with anti-MBP 1 (J, K) or 2 days (L, M) after neurectomy. Nerve degeneration (green label in inset in K) is followed by fragmentation of MBP expression in Schwann cells distal to the injury point (white dotted circle) at 24 hpn (J, K, inset in K shows both channels). At 48 hpn (L, M), MBP expression has disappeared distal to the injury site. (N-Q) Neurectomy at earlier stages, when MBP becomes expressed (at 3 pdf), showed the same results. In this case, Tg(foxD3:GFP) larvae were used to follow Schwann cells; note that despite fragmentation and loss of the MBP label, Schwann cells still remain viable as revealed by GFP expression. Scale bars: J, K, inset in N: 100 µm; B-I, L-Q, inset in E, inset in O: 50 µm. |
|
Fate of Schwann cells after neurectomy. (A) Schematic representation of the transplantation scheme used to obtain mosaic labeling of Schwann cells. Briefly, 10 to 20 donor cells of Tg(Ubi:zebrabow:cherry) blastula stage embryos were aspirated and transplanted into Tg(8.0cldnb:lynEGFP) host embryos of the same stage. The transplanted embryos were screened for the presence of red fluorescent donor cells in the pLL at 48 hpf. (B) Distribution and morphology of transplanted Schwann cells in a mock-neurectomized larva (control). The rectangle indicates the region examined in the same fish at different timepoints. (C-E) Schwann cells were examined from 1 hpn (C) to 5 dpn (E); the cells show the same distribution and general morphology through time; however, at 5 dpn (which corresponds to 8 dpf) the cell extensions form a tubular structure typical of myelinating Schwann cells (arrow). (F) A transplanted larva that was neurectomized; the rectangle indicates the region detailed in G-I. (G-I) Organization and morphology of Schwann cells over time, from 1 hpn (G) to 5 dpn (I). At 24 hpn (H) the cells exhibited vacuoles and lost their typical bipolar morphology. After 5 dpn (8 dpf) Schwann cell numbers appear reduced and the cells show thinner processes and fail to form the extensions seen in control fish (compare E to I arrows). The rectangle in H shows the region expanded in J-L. (J-L) One day after neurectomy, Schwann cells appear to engulf debris from damaged axons (arrows in L). Scale bars: B, F: 200 µm; C-E, G-I: 25 µm; J-L: 20 µm; insets in J-L: 10 µm. |
|
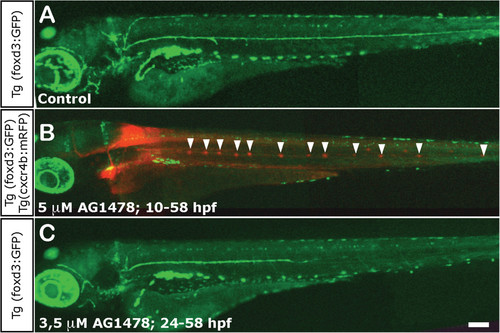
Impairment of Schwann cell migration following treatment with AG1478. (A) Normal distribution of Schwann cells in a 3 dpf control foxD3:GFP larva. (B) Total absence of Schwann cells in 3 dpf foxD3:GFP/Cxcr4:mCherry larvae treated with 5 µM AG1478 from 10 to 58 hpf (in this double transgenic, neuromasts are labeled in red fluorescence and Schwann cells in green). The supernumerary neuromasts that form in the absence of glial cells are indicated by arrowheads. (C) Partial absence of Schwann cells in 3 dpf foxD3:GFP larvae treated with 3.5 µM AG1478 from 24 to 58 hpf. With this treatement the Schwann cells migrate as far as the posterior end of the trunk (±two somites), but not into the tail at 3 dpf. Scale: A-C: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
pLL nerve regeneration in larvae totally or partially lacking Schwann cells. Transgenic tg(neuroD:EGFP) or tg(NeuroD:TagRFP) were used to evaluate the impact of Schwann cell absence on pLL nerve regeneration. (A) After 48 hpn, the regrowing nerve of control but neurectomized larvae reaches the tip of the tail. (B-F) Transgenic larvae were treated with AG1478 following two treatments: 5 µM AG1478 from 10 to 58 hpf (B-D) and 3.5 µM AG1478 from 24 to 58 hpf (E, F). In both cases, the drug was washed out at 58 hpf and the larvae were maintained in E3 medium. At 3 dpf, larvae of each treatment were separated into two groups: non-neurectomized AG-treated controls and neurectomized AG-treated larvae. Note that the nerve of control (and treated) larvae deviates from the myoseptum (dotted line) in both treatments (B, E) but deviation is more severe in the longer treatment; insets show larger magnification. In parallel, the pLL nerve of neurectomized larvae fails to regenerate properly as it defasciculates and wanders outside of the horizontal myoseptum (C, D, F), and fails to reach the tip of the tail at 48 hpn under these conditions. (G,H) At 5 dpf we quantified and compared the winding index of the nerve in neurectomized vs. non-neurectomized larvae. In both treatments, the pLL axons of neurectomized larvae have an increased winding index in comparison with their respective controls. ***P <0.001. Scale: A-F: 100 µm. |
|
pLL nerve regeneration in sdf1 mutant larvae. (A) A 3 dpf sdf1 mutant larva (also transgenic for neuroD:EGFP) shows a severely altered pLL due to failures in primordium migration during early development. The nerve is located over the yolk rather than along the horizontal myoseptum. (B) The larva was imaged immediately after neurectomy; the point of neurectomy is indicated by an arrow. Arrowhead points to an aberrant branch of the nerve that migrated towards the ventral yolk. (C) A few hours after neurectomy, the distal nerve has degenerated. (D) Twenty-four hours post neurectomy, the nerve has regenerated and follows exactly the same route as that of the original nerve, including the aberrant branch indicated in B (arrowhead). Scale: A-D: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Role of ErbB signaling in axonal regeneration. Three days post fertilization larvae were neurectomized (circles indicate position of neurectomy) after incubation with 0.05% of DMSO (controls) or 5 µM of AG1478 from 60 hpf to 5 dpf (48 hpn); this treatment does not affect Schwann cell survival but blocks ErbB signaling during the regeneration phase. Nerve regeneration in control (A-C) and drug treated larvae (D-F) was evaluated after 10 hpn, 14 hpn, and 48 hpn. The insets in C and F show the defasciculation of the regenerated nerve in treated larvae in comparison to controls. (G-I) Quantitative analysis of axonal extension after 10 hpn (G), 14 hpn (H), and 48 hpn (I) in control and AG1478 treated larvae. *P <0.05; ***P <0.001. (J) In 30% of ErbB inhibitor treated larvae, the non-neurectomized contralateral nerve appears defasciculated in comparison to the control nerve. Scale bar: C, F: 200 µm; A, B, D, E, J; insets in C and F: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
MBP expression recovery in larvae neurectomized at 3 dpf. (A) tg(foxd3:GFP) larvae were neurectomized at 3 dpf. After 48 hpn, the larvae were fixed and processed for anti-MBP labeling. At this time, MBP expression reappears in a proximal to distal wave. B: At 5 dpn, MBP expression is detected through the entire pLL nerve. Scale: A-C: 100 µm. |
|
Loss of Schwann cell differentiation markers after denervation at 5 dpf. Five-day-old tg(foxD3:GFP)/tg(NeuroD:RFP) double transgenic larvae were left untreated or were neurectomized and observed 2 dpn. In these fish, Schwann cells are labeled by green and the nerve by red fluorescence. In neurectomized fish, at 48 hpn, the regrowing nerve almost reaches the tip of the tail (arrowhead in (A), inset). At the same time, a distal decrease in GFP expression is observed (B) compared to age-matched non-neurectomized controls (C) (compare insets that show enlarged image of trunk and tail). The same experiment carried out with 3-day-old fish showed a similar result. The dotted square in (D) shows the area of the fish imaged in (E, F). E shows Schwann cells in a larva 24 hpn; F shows the same area in a control larva. Scale: E, F: 200 µm; A-C, inset in B, inset in C: 100 µm. |
|
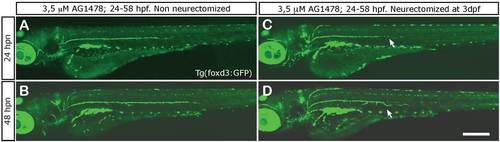
Schwann cells migrate with regenerating axons. (A, B) In non-axotomized larvae treated with 3.5 µM AG1478 from 24 to 58 hpf, Schwann cell migration is arrested and these cells remain immotile and confined to the horizontal myoseptum. (C, D) In neurectomized fish that have been treated with AG1478 as above, Schwann cells are often found at ectopic positions as they follow the erratic path of the regrowing nerve. Scale: A-D: 200 µm. |