- Title
-
Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration
- Authors
- Schindler, Y.L., Garske, K.M., Wang, J., Firulli, B.A., Firulli, A.B., Poss, K.D., Yelon, D.
- Source
- Full text @ Development
|
hand2 overexpression increases cardiomyocyte production. (A-D) In situ hybridization depicts cmlc2 expression at 18 somites in (A) wild-type embryos, (B) hand2-overexpressing embryos (hand2 OE), (C) embryos expressing a DNA binding-deficient form of hand2 (hand2 EDE) and (D) embryos expressing a dimerization-deficient form of hand2 (hand2 P); dorsal views, anterior upwards. Broader expression of cmlc2 is found within the ALPM of embryos overexpressing hand2 or hand2 EDE. (E) Average area of cmlc2 expression, in arbitrary units, in wild-type embryos and in embryos overexpressing hand2, hand2 EDE or hand2 P. Error bars indicate s.d.; asterisks indicate significant differences from wild type. Overexpression of hand2 or hand2 EDE increases area of cmlc2 expression (n=13-25; *P<0.001), whereas overexpression of hand2 P does not (n=11; P=0.87). (F) Bar graph compares average number of cardiomyocytes at 36hpf in wild-type embryos and in embryos overexpressing hand2. Error bars indicate s.d.; asterisk indicates a significant increase compared with wild type (n=17-19; *P<0.001). EXPRESSION / LABELING:
|
|
Influence of Hand2 on cardiomyocyte production is dependent on dimerization and not on direct DNA binding. (A-E) In situ hybridization depicts cmlc2 expression at 21 somites in (A) wild-type embryos, (B) hans6 mutant embryos, (C) hans6 mutant embryos injected with hand2 mRNA, (D) hans6 mutant embryos injected with hand2 EDE mRNA and (E) hans6 mutant embryos injected with hand2 P mRNA; dorsal views, anterior upwards. The hans6 mutation is a null allele of hand2 resulting from a deletion that removes the entire hand2 gene (Yelon et al., 2000). At this stage, wild-type cardiomyocytes have formed a ring (A), whereas few cardiomyocytes have formed in the hans6 mutants (B). Cardiomyocyte formation is substantially rescued with the injection of hand2 (C) or hand2 EDE (D) mRNA, but not of hand2 P mRNA (E). (F) Average area of cmlc2 expression, as in Fig. 1E. Asterisks indicate significant differences between the phenotype of hans6 mutant embryos and the phenotypes of hans6 mutant embryos overexpressing versions of hand2. Overexpression of hand2 or hand2 EDE partially rescues the area of cmlc2 expression in hans6 mutants (n=14; *P<0.001), whereas hand2 P does not (n=14; P=0.085). EXPRESSION / LABELING:
|
|
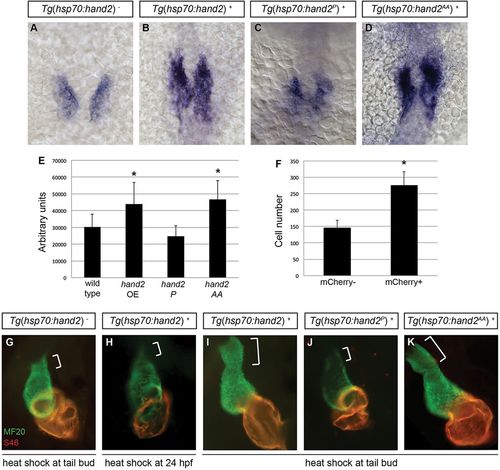
Cardiac expansion induced by hand2 overexpression requires phosphorylation-independent dimerization of Hand2. (A-D) In situ hybridization depicts cmlc2 expression at 18 somites in (A) nontransgenic embryos, (B) Tg(hsp70:hand2) embryos, (C) Tg(hsp70:hand2P) embryos and (D) Tg(hsp70:hand2AA) embryos, heat-shocked at 10hpf; dorsal views, anterior upwards. (E) Average area of cmlc2 expression, as in Fig. 1E, in nontransgenic and transgenic embryos, following heat shock at 10hpf. Asterisks indicate significant differences from nontransgenic embryos. Overexpression of hand2 or hand2 AA increases the area of cmlc2 expression (n=15-32; *P<0.001), whereas hand2 P does not (n=13; P=0.05). (F) Average number of cardiomyocytes at 36hpf, as in Fig. 1F, in nontransgenic embryos and in Tg(hsp70:hand2) embryos, following heat shock at 10hpf. Asterisk indicates a significant difference from nontransgenic embryos (n=16-20; *P<0.001). (G-K) Immunofluorescence at 36hpf for MF20 (green, visible in the ventricle) and S46 (red, visible in the atrium) shows cardiac morphology. Frontal views; brackets mark the outflow tract. Hearts of Tg(hsp70:hand2) embryos heat-shocked at 24hpf (H) resemble nontransgenic hearts heat-shocked at 10hpf (G). Tg(hsp70:hand2) embryos heat-shocked at 10hpf (I) show an overall increase in cardiac size and an enlarged outflow tract. Similar morphology is seen after heat shock at 10hpf in Tg(hsp70:hand2AA) embryos (K), but not in Tg(hsp70:hand2P) embryos (J). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Overexpression of hand2 causes outflow tract expansion. (A-H) MF20 antibody staining (red) and mef2cb in situ hybridization (green) at 36hpf in (A,B) nontransgenic, (C,D) Tg(hsp70:hand2), (E,F) Tg(hsp70:hand2P) and (G,H) Tg(hsp70:hand2AA) embryos, all of which were heat-shocked at 10hpf. Frontal views; (A,C,E,G) partial reconstructions of confocal z-stacks with arrows indicating outflow tract; (B,D,F,H) single slices with brackets marking outflow tract. Embryos overexpressing hand2 (C,D) or hand2 AA (G,H) exhibit expanded populations of mef2cb-expressing cells, whereas embryos overexpressing hand2 P (E,F) do not. EXPRESSION / LABELING:
|
|
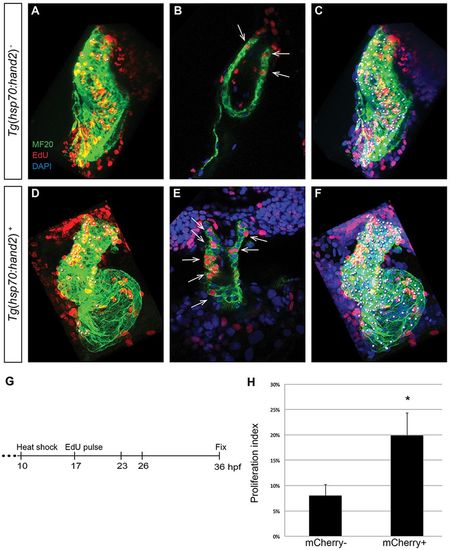
Increased proliferation contributes to cardiac expansion in hand2-overexpressing embryos. (A-F) EdU incorporation in hearts of (A-C) nontransgenic and (D-F) Tg(hsp70:hand2) embryos at 36hpf, following heat shock at 10hpf and EdU pulse at 17hpf. Partial reconstructions of confocal z-stacks with ventricle upwards (A,C,D,F) and representative single slices (B,E). (A,D) White dots indicate EdU-positive (red) cells that are also MF20-positive (green) differentiated cardiomyocytes. (B,E) Arrows indicate EdU-positive cells that are also MF20 positive; DAPI (blue) marks all nuclei. (C,F) White dots indicate all myocardial nuclei. Qualitative assessment suggests increased numbers of EdU-positive cardiomyocytes in hand2-overexpressing hearts (D,E), particularly in the distal region of the ventricle and the outflow tract. (G) Timeline of experimental design. (H) Proliferation indices in nontransgenic (mCherry-negative) and Tg(hsp70:hand2) (mCherry-positive) embryos; error bars indicate s.d. Proliferation index was calculated by dividing the number of EdU-positive cardiomyocytes by the total number of cardiomyocytes. A significant increase in proliferation index was evident in hand2-overexpressing embryos (n=8 or 9; *P<0.001). EXPRESSION / LABELING:
|
|
Overexpression of hand2 increases proliferation of SHF-derived progenitor cells. (A-D) EdU incorporation in (A,B) nontransgenic and (C,D) Tg(hsp70:hand2) embryos at 26hpf, following heat shock at 10hpf and EdU pulse at 17hpf; partial reconstructions of confocal z-stacks with ventricle upwards. (A,C) White dots indicate EdU-positive (red) cells that are MF20 positive (green) and/or expressing Tg(nkx2.5:ZsYellow) (yellow). (B,D) White dots indicate all nuclei (DAPI, blue) of cells that are MF20 positive and/or expressing Tg(nkx2.5:ZsYellow). (E) Timeline of experimental design. (F) Proliferation indices, as in Fig. 5H, for two populations of cells: SHF-derived cells, defined as Tg(nkx2.5:ZsYellow)-expressing cells with very low or no MF20 staining; and FHF-derived cells, defined as cells with clearly detectable MF20 staining. Proliferation index was calculated for each population independently by dividing the number of EdU-positive cells by the total number of cells in the population. A significant increase in proliferation index was evident in the SHF-derived cells in hand2-overexpressing embryos (n=10 or 11; *P=0.003), but not in the FHF-derived cells (n=10 or 11; P=0.528). EXPRESSION / LABELING:
|
|
hand2 overexpression limits expression of blood and vessel genes within the ALPM. (A-L) In situ hybridization depicts expression of (A,B) scl, (C,D) etsrp, (E,F) pu.1, (G,H) mef2cb, (I,J) nkx2.5 and (K,L) gata4 in (A,C,E,G,I,K) nontransgenic embryos and (B,D,F,H,J,L) Tg(hsp70:hand2) embryos following heat shock at 10hpf. Dorsal views, anterior upwards, at (A-J) 12 somites and (K,L) 10 somites. Overexpression of hand2 results in (A-F) decreased distribution of scl, etsrp and pu.1 expression, as well as (G,H) increased expression of mef2cb. By contrast, expression of (I,J) nkx2.5 and (K,L) gata4 appear relatively normal in hand2-overexpressing embryos at these stages. EXPRESSION / LABELING:
|
|
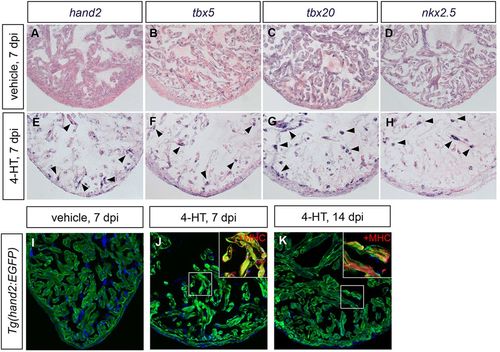
Cardiac injury induces hand2 expression. (A-H) In situ hybridization depicts expression of (A,E) hand2, (B,F) tbx5, (C,G) tbx20 and (D,H) nkx2.5 in ventricles of adult Z-CAT fish 7days post-injection (dpi) of vehicle (A-D) or 4-HT (E-H). Expression of each gene is enhanced in spared myocardium following injury (E-H, arrowheads). (I-K) Expression of Tg(hand2:EGFP) (green) highlights the activation of hand2 regulatory sequences following injury. Images are single confocal slices of ventricular tissue at 7dpi (I,J) and 14dpi (K); insets demonstrate myosin heavy chain (MHC; red) localization in hand2-expressing cells. |
|
Overexpression of hand2 boosts cardiomyocyte proliferation in response to injury. (A) Schematic representation of transgenes used for cardiomyocyte ablation and myocardium-specific hand2 overexpression in Z-CAT fish. (B) Proliferation indices for ventricular cardiomyocytes; error bars indicate s.e.m. Proliferation was assessed 7days after 4-HT injection in Z-CAT fish with or without induced cardiomyocyte-specific hand2 overexpression. hand2-overexpressing fish exhibited significantly increased proliferation following ablation injury (n=9 or 10; *P<0.05). (C) Proliferation indices in injured cmlc2:CreER; β-act2:RSH fish; error bars indicate s.e.m. Proliferation was assessed at the resection plane 7days post-amputation (dpa) in animals that were injected with either vehicle or 4-HT 5days before injury. hand2-overexpressing fish exhibited significantly increased proliferation following resection injury (n=4 or 5; *P<0.005). (D,E) Representative examples of cardiomyocyte proliferation at 7dpa at the resection plane (brackets) in injured cmlc2:CreER; β-act2:RSH fish that were treated with vehicle (D) or 4-HT (E). Mef2 (green) marks cardiomyocytes and PCNA (red) marks proliferating cells; arrows in insets indicate double-positive cells. |
|
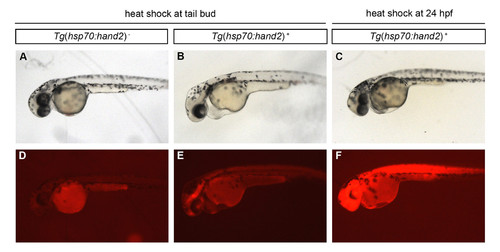
Induction of hand2 overexpression at 10 hpf, but not at 24 hpf, produces a morphologically evident cardiac phenotype. (A-F) Lateral views of live embryos at 36 hpf depict (A-C) embryonic morphology and (D-F) mCherry fluorescence in (A,D) a nontransgenic embryo and (B,C,E,F) embryos carrying the transgene Tg(hsp70:FLAG-hand2-2A-mCherry) (abbreviated as Tg(hsp70:hand2)), after heat shock at (A,B,D,E) 10 hpf (tail bud) or (C,F) 24 hpf. Heat shock of transgenic embryos at 10 hpf leads to pericardial edema (B), and residual mCherry fluorescence is still evident in these embryos at 36 hpf (E). In contrast, edema is not observed in transgenic embryos after heat shock at 24 hpf (C); mCherry fluorescence remains pervasive in these embryos at 36 hpf (F). |
|
Levels of protein production in different transgenic lines. (A-H) Lateral views of live embryos at 30 hpf depict bright field images of representative embryos from different transgenic lines (A-D), along with corresponding mCherry fluorescence (E-H). (E) No mCherry fluorescence is induced in heat-shocked nontransgenic embryos. (F-H) mCherry fluorescence is readily detectable in representative embryos carrying the transgenes (F) Tg(hsp70:FLAG-hand2-2A-mCherry) (Tg(hsp70:hand2)), (G) Tg(hsp70:FLAG-hand2P-2A-mCherry) (Tg(hsp70:hand2P)), and (H) Tg(hsp70:FLAG-hand2AA-2A-mCherry) (Tg(hsp70:hand2AA)). (I) Western blot analysis compares levels of FLAG-tagged Hand2 protein in different transgenic lines. Embryos were deyolked and lysates were prepared as previously described (Link et al., 2006), and blots were probed with either a monoclonal anti-FLAG M2 antibody (F1804, Sigma, 1:2000) or a monoclonal anti-α-Tubulin antibody (T6728, Sigma, 1:10,000), followed by a rabbit anti-mouse IgG HRP-conjugated secondary antibody (ab97046, Abcam, 1:10,000). Proteins were visualized using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific). Each lane contains lysate from 15 embryos at 36 hpf (2 hours following heat shock), and the lanes compare protein levels in nontransgenic embryos (WT), Tg(hsp70:hand2) embryos (hand2), Tg(hsp70:hand2P) embryos (hand2P), and Tg(hsp70:hand2AA) embryos (hand2AA). All three transgenic lines contain a ~23 kD FLAG-Hand2 protein, with comparable levels in Tg(hsp70:hand2) and Tg(hsp70:hand2AA) embryos and lower levels in Tg(hsp70:hand2P) embryos. |
|
Normal islet1 expression at the venous pole in embryos overexpressing hand2. (A-B) Immunofluorescence at 36 hpf for MF20 (red, visible throughout the heart) and Islet1 (green, visible in the nuclei of a subset of atrial cells). Frontal views, dorsal to the top; arrows point to the Islet1-positive population at the venous pole of the atrium. Following heat shock at 10 hpf, the population of Islet1-positive cells at the venous pole appears similar in hearts from (A) nontransgenic embryos and (B) Tg(hsp70:hand2) embryos. (C) Bar graph compares average number of Islet1-positive cardiomyocytes at 36 hpf in nontransgenic embryos and Tg(hsp70:hand2)embryos, following heat shock at 10 hpf. Error bars indicate standard deviation; no significant difference is observed between these two data sets (n=12, p=0.09). (D-E) In situ hybridization depicts islet1 expression at 36 hpf, following heat shock at 10 hpf. Frontal views, dorsal to the top; arrows point to the ring of islet1-expressing cells at the venous pole of the atrium. Expression patterns are similar in (D) nontransgenic embryos and (E) Tg(hsp70:hand2) embryos. |
|
No evident influence of hand2 overexpression on cardiomyocyte proliferation after initial heart tube assembly. (A-F) EdU incorporation in hearts of (A-C) nontransgenic and (D-F) Tg(hsp70:hand2) embryos at 36 hpf, following heat shock at 10 hpf and EdU pulse at 23 hpf; (A,C,D,F) partial reconstructions of confocal z-stacks with ventricle up and (B,E) representative single slices. Dots, arrows, and color schemes are as described for Fig. 5A-F. Note that the nontransgenic heart shown (A) contains a number of EdU-positive blood cells that were trapped during fixation; EdU-positive blood cells are less commonly observed within the hearts of hand2-overexpressing embryos (D), due to their impaired circulation. (G) Timeline of experimental design. (H) Bar graph compares proliferation indexes in nontransgenic (mCherry-negative) and Tg(hsp70:hand2) (mCherry-positive) embryos, as in Fig. 5H. No change in proliferation index is seen in hand2-overexpressing embryos (n=8-11; p=0.196). |
|
No evident influence of hand2 overexpression on cardiomyocyte proliferation within the heart tube at 23 hpf. (A-D) EdU incorporation in hearts of (A,B) nontransgenic and (C,D) Tg(hsp70:hand2) embryos at 23 hpf, following heat shock at 10 hpf and EdU pulse at 17 hpf; partial reconstructions of confocal z-stacks. Images depict the elongating cardiac cone, positioned with its arterial end toward the top. (A,C) White dots indicate EdU-positive (red) cells that are also MF20-positive (green) differentiated cardiomyocytes. (B,D) White dots indicate all nuclei (DAPI, blue) of myocardial cells, including both EdU-positive and EdU-negative cardiomyocytes. (E) Timeline of experimental design. (F) Bar graph compares proliferation indexes in nontransgenic (mCherry- negative) and Tg(hsp70:hand2) (mCherry-positive) embryos, as in Fig. 5H. No change in proliferation index is seen in hand2- overexpressing cardiomyocytes (n=10-11; p=0.252). Similarly, when we assessed EdU incorporation in hand2-overexpressing embryos at 26 hpf, following heat shock at 10 hpf and EdU pulse at 14 hpf, we did not see an increased proliferation index in hand2- overexpressing cardiomyocytes (proliferation index of 28 ± 4% in nontransgenic embryos compared to proliferation index of 27 ± 4% in hand2-overexpressing embryos; n=7-10, p=0.58). |
|
Endocardium is present in embryos overexpressing hand2. (A-H) Lateral views of live embryos at 30 hpf depict (A-B) embryonic morphology and (C-H) expression of the transgene Tg(kdrl:GRCFP) in the vasculature of (A,C,E,F) nontransgenic embryos and (B,D,G,H) Tg(hsp70:hand2) embryos, following heat shock at 10 hpf. (C-D) General vascular patterning and sprouting of intersomitic vessels (inset) is intact in embryos overexpressing hand2 (D). (E-H) Partial reconstructions of confocal z-stacks (E,G) and representative single slices (F,H) highlight the endocardium (arrows): just as in nontransgenic embryos (E,F), a thin layer of endocardial tissue lines the entire heart tube in embryos overexpressing hand2 (G,H). |