- Title
-
A loss of function screen of identified genome-wide association study Loci reveals new genes controlling hematopoiesis
- Authors
- Bielczyk-Maczyńska, E., Serbanovic-Canic, J., Ferreira, L., Soranzo, N., Stemple, D.L., Ouwehand, W.H., Cvejic, A.
- Source
- Full text @ PLoS Genet.
|
In vivo morpholino screen in zebrafish identifies 15 new regulators of thrombopoiesis. MOs were injected into one-cell stage transgenic Tg(cd41:EGFP) zebrafish embryos and assayed for their effect on the number of thrombocytes (cd41high) at 3 dpf. Representative confocal images were taken of the CHT. For akap10, brd3a, brf1b, kalrn 1, kalrn 2, kif1b, mfn2, pdia5, psmd13 and satb1 a severe decrease in the number of cd41high positive cells was observed. brf1a, rcor1, waspla, wasplb and wdr66 depletion resulted in a mild phenotype, and fen1, grtp1b and tmcc2 MO injected embryos showed no phenotype. All embryos are oriented with anterior to the left and dorsal to the top. White arrow – thrombocytes; white arrowhead – HSCs. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Characterization of HSCs in candidate gene depleted embryos. To assess the stage at which hematopoiesis of each MO injected embryo was defective, we performed whole mount in situ hybridization using a c-myb probe at 3 dpf. Although more than half of MOs had no effect on the number of HSCs, depletion of rcor1 resulted in increased numbers of HSCs and depletion of kalrn1, kalrn2, mfn2, pdia5, psmd13 and wasplb resulted in decreased numbers of HSCs in CHT at 3 dpf. Representative images of CHT region are shown. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
brd3a is an important regulator of thrombopoiesis. (A) Live confocal imaging of zebrafish embryos injected with hBRD3-GFP mRNA revealed the nuclear localization of hBRD3 and that it binds to mitotic chromosomes (white arrows). Expression of hBRD3 in brd3a MO injected embryos resulted in a partial rescue of the number of thrombocytes as shown in (B). (C) A graph to illustrate the number of thrombocytes in control, splice brd3a MO and splice brd3a MO plus hBRD3 mRNA injected embryos. Each dot represents the number of thrombocytes in the individual MO-injected embryos with respect to control. A blue horizontal line represents the mean value of the number of thrombocytes for each group of embryos. Student t test, * p = 0.016; n = 17. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
brd3a is required for differentiation and not maintenance of thrombocytes. (A) Exposure of zebrafish embryos to 1 μg/ml (+)-JQ1 from 24 hpf resulted in a severe reduction in the number of thrombocytes compared to DMSO or (-)-JQ1 treated embryos. (B) A graph to illustrate the average number of thrombocytes at 3 dpf. Embryos were exposed to DMSO, (-)-JQ1 or (+)-JQ1 starting from 24 hpf. Each dot represents thrombocyte count for a single embryo. Horizontal line denotes average thrombocyte number. Student t test, ** p = 1.6×10-8, n = 17. (C) To assess whether brd3a is essential for differentiation of thrombocytes or for their maintenance and survival we incubated Tg(cd41:EGFP) embryos in DMSO or with (+)-JQ1 inhibitor starting from 3 dpf and checked thrombocyte number 24 hours later, at 4 dpf. We found that in untreated (DMSO) embryos the number of thrombocytes markedly increased between 3- and 4 dpf; however, in (+)-JQ1 treated embryos there was no change in the number of thrombocytes. In contrast, the inhibitor had no adverse effect on the number of HSCs during this 24 h period of treatment (D). Representative confocal images of CHT region are shown. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
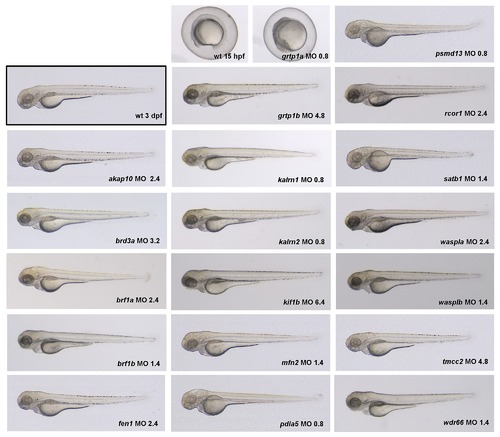
In order to determine the optimal dose of the MOs to be injected, a dose-response experiment was performed. For each gene the concentration of MO (shown in lower right corner of each image, in ng) was selected that elicited a specific phenotype without an overt non-specific effect. Knock-down of grtp1a resulted in early embryonic lethality even when injected with 0.8 ng of MO. Thus, grtp1a was excluded from further analysis. The optimal MO dose for each gene as determined in this experiment was used in all subsequent experiments. |
|
Whole-mount in situ hybridization using brd3a, brf1b, waspla, kalrn1 and wdr66 riboprobes is shown. Panels show a lateral view of embryos at 3 dpf. All embryos are oriented with anterior to the left and dorsal to the top. |
|
To verify that MOs used in this study exerted a specific effect on hematopoiesis we designed a second non-overlapping morpholino (MO2) for all “phenotypic” candidate genes. Splice modifications caused by gene-specific MOs were assayed by RT-PCR, using gene-specific primers, and were confirmed for all but brf1a, kif1b and wasplb MO2. These three MO2 were therefore excluded from further analysis. Injection of the remaining 12 MO2 resulted in the phenotype that was comparable to the one observed with the first MO. Representative fluorescent images of the CHT are shown. All embryos are oriented with anterior to the left and dorsal to the top. |
|
MO activities in zebrafish embryos include both sequence-specific RNA binding as well as effects not associated with loss of function of the targeted locus i.e. “off-target” effects. As p53 MO can reduce common off-target effects, the number of thrombocytes was assessed in CHT at 3 dpf in embryos co-injected with p53 MO and gene-specific MOs. Concurrent knock down of p53 with gene-specific MOs did not attenuate the thrombocyte phenotype induced by gene-specific MOs, confirming that the observed decrease in the number of thrombocytes was not induced by off-target effects of MOs. All embryos are oriented with anterior to the left and dorsal to the top. |
|
A) Fish heterozygous for a mutation in brf1b (allele sa3097, ZMP) were crossed and their progeny (n = 41) was subjected to a clotting time assay at 5 dpf. The time of clotting after caudal vein puncture was recorded and the larvae were genotyped. Each bar represents average clotting time in the group, SEM is shown. One-tailed Student t test, * p = 0.042. B) A graph to illustrate the number of thrombocytes in control (n = 33), splice rcor1 MO (n = 28) and splice rcor1 MO plus rcor1 mRNA (n = 15) injected embryos. Each bar represents average number of thrombocytes in the group, SEM is shown. One-tailed Student t test, * p = 0.024. C) Representative fluorescent images of embryos in the rcor1 rescue experiment. All embryos are oriented with anterior to the left and dorsal to the top. |
|
To assess the HSC emergence in the aorta-gonad-mesonephros (AGM) region, we injected gene-specific MOs in single-cell stage Tg(c-myb:GFP) transgenic embryos. For kalrn1, mfn2, pdia5, psmd13 and wasplb MO injected embryos no difference in the number of HSCs was observed when compared to the control at 30 hpf. However, kalrn2 and rcor1 depleted embryos had a marked decrease in the number of HSCs at 30 hpf, implying an important role of these genes in specification of HSCs in the AGM. Representative images of the AGM region are shown. All embryos are oriented with anterior to the left and dorsal to the top. |
|
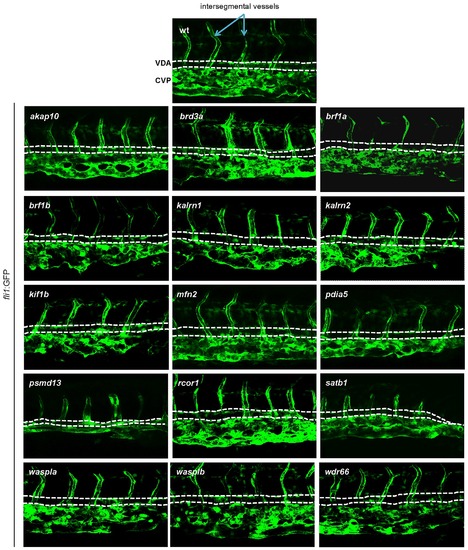
In order to assess vascular development, MOs targeting candidate genes were injected into Tg(fli1:EGFP) embryos. Vascular morphology of the embryos was assessed at 3 dpf. No major abnormalities in vascular morphogenesis were observed for any of the tested MOs, indicating that the hematopoietic defects were not secondary to a vascular phenotype. Representative images of the CHT region are shown. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
|
|
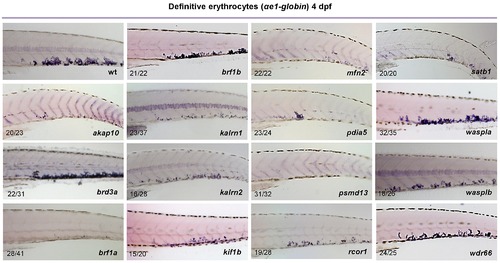
Whole mount in situ hybridization was performed using a probe specific to αe1-globin at 4 dpf. As a result of candidate gene knock down, depletion of αe1-globin staining was observed for all genes but brd3a, brf1b, waspla and wdr66. Representative images of CHT are shown. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
|
|
All the erythrocytes present in circulation of 2 dpf zebrafish embryos derive from the primitive wave of hematopoiesis. In order to assess the primitive erythropoiesis, MO injected embryos were stained with O-dianisidine at 2 dpf (arrow). Knockdown of 12 out of 15 candidate genes, namely: akap10, brf1a, brf1b, kalrn1, mfn2, pdia5, psmd13, rcor1, satb1, waspla, wasplb and wdr66, resulted in no observable phenotype. Depletion of brd3a, kalrn2 and kif1b resulted in a severe reduction in the number of primitive erythrocytes. All the embryos are positioned anterior up and dorsal to the back. |
|
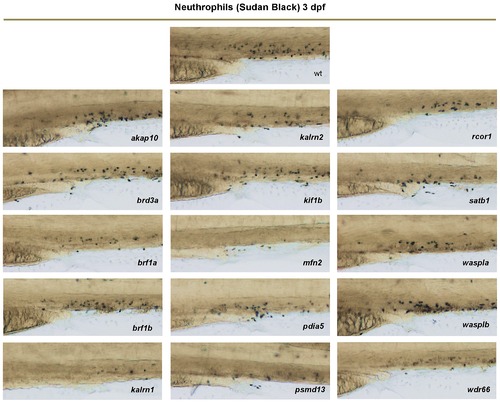
Individual neutrophils may be detected with Sudan Black staining. The number of neutrophils in MO-injected embryos (nembryos = 15) in CHT was counted at 3 dpf. Knock-down of akap10, brd3a, brf1b, kif1b, waspla or wasplb caused no phenotype. A mild decrease (20–50%) in the number of neutrophils was observed for brf1a, kalrn2, pdia5, rcor1, satb1 and wdr66 MO injected embryos compared to control and kalrn1, mfn2 and psmd13 MO injected embryos showed a severe (over 50%) depletion of neutrophils when compared to control. Representative images of the CHT region at 3 dpf are shown. All embryos are oriented with anterior to the left and dorsal to the top. PHENOTYPE:
|
|
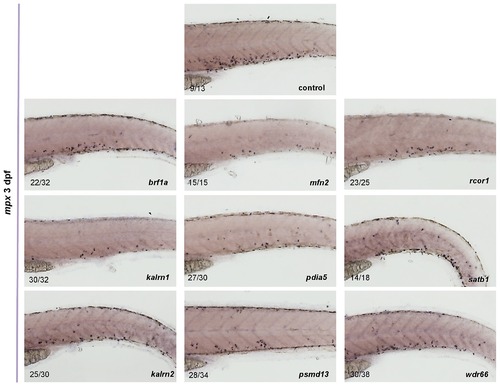
The expression of mpx in control as well as in brf1a, kalrn1, kalrn2, mfn2, pdia5, psmd13, rcor1, satb1 and wdr66 depleted embryos was assessed by in situ hybridization. For all genes tested the reduced number of mpx positive cells was observed when compared to the control. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
|
|
In order to detect macrophages, whole mount in situ hybridization was performed using the probe specific to mpeg1 at 3 dpf. For 13 out of 15 tested MOs (akap10, brd3a, brf1a, brf1b, kalrn1, kalrn2, mfn2, pdia5, psmd13, rcor1, satb1, wasplb and wdr66) there was no observable difference in the number of macrophages. However, knock down of kif1b and waspla resulted in a severe reduction in the number of macrophages in CHT at 3 dpf. All embryos are oriented with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
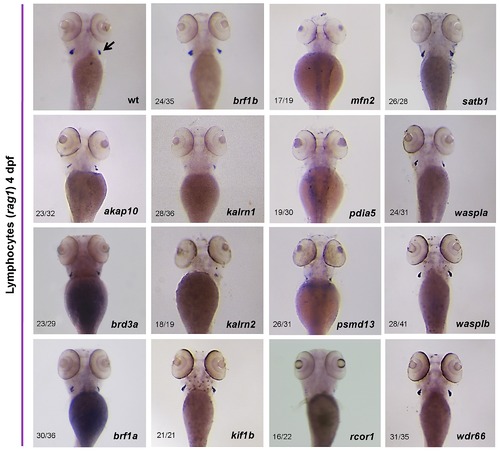
Differentiated thymic T-cells could be readily identified by rag1 expression when examined at 4 dpf. Whole mount in situ hybridization with a rag1 riboprobe revealed a severe decrease in the number of T lymphocytes in kalrn2, mfn2, pdia5 and psmd13 MO injected embryos in thymi (arrow) at 4 dpf. Knock down of akap10, kalrn1 and rcor1 caused a moderate decrease in T lymphocyte numbers. All the embryos are positioned anterior up and dorsal to the back. |
|
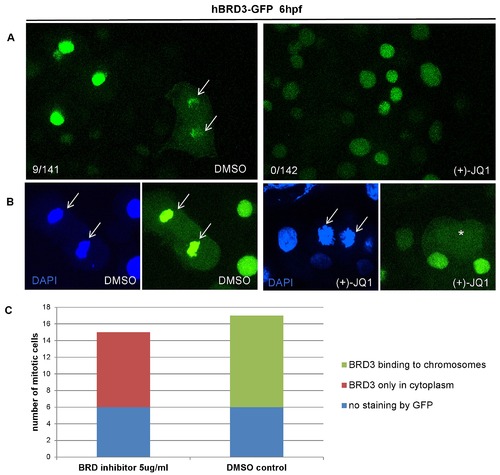
(+)-JQ1 is a highly specific inhibitor, which displaces BET proteins from chromatin by competitively binding to the acetyl-lysine recognition pocket of BET bromodomains. (A) Treatment of zebrafish embryos with (+)-JQ1 disrupted the chromatin occupancy of hBRD3-GFP as demonstrated by the absence of GFP-positive mitotic chromosomes in these embryos (0/142 GFP-positive cells) compared to 9/141 GFP-positive cells in the DMSO control group. (B–C) To further quantify the chromatin occupancy of hBRD3-GFP during mitosis, we selected ≥15 mitotic cells, as shown by DAPI staining, and counted how many of these cells were GFP positive in the presence of (+)-JQ1 or DMSO (control). Whereas in (+)-JQ1 treated embryos none of the DAPI positive mitotic chromosomes were GFP positive, in DMSO treated embryos 11 cells were double DAPI/GFP positive. Asterisk depicts GFP in the cytoplasm and arrow shows mitotic chromosomes. |
|
To assess the effect of the inhibitor on embryo development, dose response experiments were performed. Embryos treated with 1 μg/mL (+)-JQ1 from 6 hpf died by 24 hpf. The same concentration of the inactive enantiomer ()-JQ1 did not affect the development of embryos. Incubation in lower concentration of (+)-JQ1 (0.5 μg/ml) led to aberrant development, i.e. tail malformation and heart edema. The lowest tested dose, 0.25 μg/ml, did not affect the development of the embryos. To avoid early embryonic lethality (+)-JQ1 inhibitor was added at 24 hpf. The embryos exhibited overall normal development even at the higher concentration (1 μg/ml) of (+)-JQ1. Representative images of embryo morphology are shown, taken at 24 hpf, 48 hpf and 72 hpf. All the embryos are positioned with anterior to the left and dorsal to the top. |
|
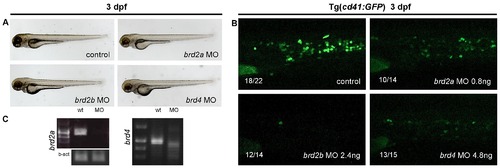
To assess the role of other BET family members in thrombopoiesis MO knock down of brd2a, brd2b and brd4 was performed. A) Although morphologically normal, embryos injected with brd2a, brd2b or brd4 MOs had a severe decrease in the number of thrombocytes at 3 dpf (B). Representative pictures of CHT are shown. C) For splice-blocking MOs the effect of the MOs was confirmed by RT-PCR. All the embryos are positioned with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|