- Title
-
Pkd1 Regulates Lymphatic Vascular Morphogenesis during Development
- Authors
- Coxam, B., Sabine, A., Bower, N.I., Smith, K.A., Pichol-Thievend, C., Skoczylas, R., Astin, J.W., Frampton, E., Jaquet, M., Crosier, P.S., Parton, R.G., Harvey, N.L., Petrova, T.V., Schulte-Merker, S., Francois, M., Hogan, B.M.
- Source
- Full text @ Cell Rep.
|
lyc1 Mutants Display Reduced Lymphatic Development (A and B) Overall morphology of wild-type siblings (A) and lyc1 mutants (B) at 4 dpf. (C and D) The vasculature Tg(fli1a:EGFPy1; flt1:tomatohu5333Tg) of (C) wild-type (WT) (arrowheads indicate thoracic duct) and (D) lyc1 mutants at 4 dpf (asterisks indicate absence of thoracic duct). (E and G) The vasculature Tg(fli1a:EGFPy1; flt1:tomatohu5333Tg) in wild-type sibling (E) and mutant embryos (G) at 56 hpf (arrows indicate lymphatic precursors known as parachordal lymphangioblasts, PLs). (F and H) flt1:tomatohu5333Tg expression marks the arterial ECs, a loss of signal (brackets) indicating venous intersegmental vessels (vISVs). (I–K) Quantification of (I) thoracic duct extent across ten somites (WT n = 40, lyc1 n = 17), (J) parachordal lymphangioblasts (WT n = 78, lyc1 n = 17), and (K) venous sprouts (WT n = 40, lyc1 n = 15). DA, dorsal aorta; PCV, posterior cardinal vein. Error bars indicate SEM. See also Figures S1 and S2. |
|
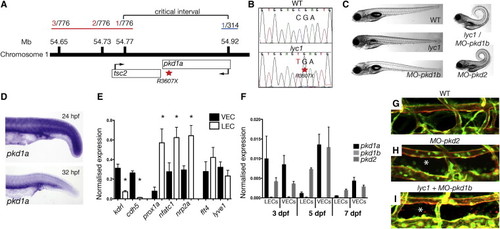
lyc1 Is a pkd1a Mutant (A) Overview of positional cloning of lyc1. Individual recombinant embryos (labeled in red left [from 776 embryos analyzed], labeled in blue right [from 314 embryos analyzed]) identify flanking polymorphic markers and limit the critical interval to a region containing partial sequences for pkd1a and tsc2. (B) Sequence chromatograms showing the wild-type (upper) and pkd1a mutant (R3607X, lower) sequences. (C) Overall morphology of 5 dpf WT, lyc1, MO-pkd1b, lyc1/MO-pkd1b, and MO-pkd2 embryos. The injection of MO-pkd1b into lyc1 mutants recapitulates the published MO-pkd1a/1b double loss-of-function phenotype ( Mangos et al., 2010). (D) Expression pattern of pkd1a by in situ hybridization in the trunk of wild-type zebrafish at 24 hpf and 32 hpf. (E) Quantitative RT-PCR for markers enriched in venous endothelial cells (VECs); kdrl, cdh5, LECs; prox1a, nfatc1, nrp2a; and both flt4 and lyve1 demonstrated the purity of FACS-isolated populations at 5 dpf. (F) Quantitative RT-PCR for pkd1a, pkd1b, and pkd2 transcripts in 3, 5, and 7 dpf VEC and LEC populations. (G–I) The vasculature of 5 dpf WT, lyc1/MO-pkd1b and MO-pkd2 embryos (5 and 7.5 ng MO, respectively); asterisk indicates absence of thoracic duct in Tg(fli1a:EGFPy1; kdrl:egfps843) embryos. Error bars indicate SEM. See also Figures S3 and S4. |
|
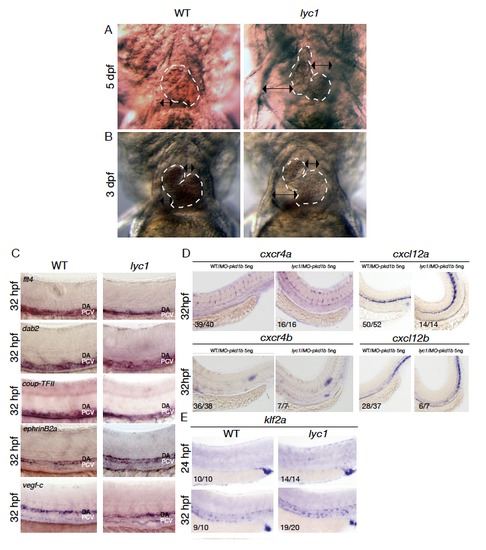
Further phenotypic analysis of lyc1. Related to Figure 1 (A-B) Representative overall morphology of WT and lyc1 mutant embryonic hearts and pericardial cavities at 3 (A) and 5 dpf (B). Double-sided arrows indicate the distance between the pericardium and heart. Dashed line indicates the outline of the myocardium. (C) In situ hybridization analysis of flt4 (n=22), dab2 (n=16), coup-TFII (n=20), ephrinb2a (n=17) and vegf-c (n=28) in lyc1 mutant embryos revealed no alterations in normal expression patterns. N values indicate the total number of embryos examined from an incross of known heterozygotes (expected 25% lyc1 mutants). Individual genotype confirmed mutant embryos are shown in the right hand panels. (D) Expression of cxcr4a was unchanged at 32 hpf in control WT/MO-pkd1b (embryos show no phenotype after pkd1b knockdown only and internally control for MO toxicity) (n=39/40) compared with phenotypically mutant (based on body curvature in the presence of MO-pkd1b) lyc1/MO-pkd1b embryos (5ng MO) (n=16/16). Expression of cxcr4b was unchanged at 32 hpf in control (WT/MO-pkd1b) (n=36/38) compared with phenotypically mutant lyc1/MO-pkd1b embryos (5ng MO) (n=7/7). Expression of cxcl12a was unchanged at 32 hpf in control (WT/MO-pkd1b) (n=50/52) compared with phenotypically mutant lyc1/MO-pkd1b embryos (5ng MO) (n=14/14). Expression of cxcl12b was unchanged at 32 hpf in control (WT/MO-pkd1b) (n=28/37) compared with phenotypically mutant lyc1/MO-pkd1b embryos (5ng MO) (n=6/7). (E) Expression of klf2a at 24 (n=10/10) and 32 hpf (n=9/10) is normal in WT embryos. Expression of klf2a at 24 hpf (n=14/14) and 32 hpf (n=19/20) in lyc1 mutant embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Parachordal lymphangioblasts fail to directionally migrate and display altered cell dynamics in the lyc1 mutant. Related to Figure 1. (A-B) Schematic overview of the movement of the leading front of individual parachordal lymphangioblasts (t=10.5h) from 56 hpf (scale bar: 10 μm) in (A) WT/MO-pkd1b (5ng MO) (n=3 embryos, n=4 leading fronts) and (B) phenotypically mutant lyc1/ MO-pkd1b (5ng MO) (n=5 embryos, n=5 leading fronts). (C-D) Quantification of (C) movement of cell front and (D) origin to endpoint of cell front distance migrated by parachordal lymphangioblasts (t=10.5h) from 56 hpf in in WT/MO-pkd1b (5ng MO) (n=3 embryos, n=4 leading front) and phenotypically mutant lyc1/ MO-pkd1b (5ng MO) (n=5 embryos, n=5 leading fronts) (μm). (E) Quantification of duration of individual filipodial extensions in single parachordal lymphangioblasts at the horizontal myoseptum by time-lapse imaging (spinning disc). WT/MO-pkd1b (n=3) and phenotypically mutant lyc1/MO-pkd1b embryos (n=4) (5ng MO) were examined between 56-64 hpf (1 time unit = 4 minutes). (F) Quantification of the number of filipodial extensions per parachordal lymphangioblasts by time-lapse imaging (spinning disc) in WT/MO-pkd1b (n=3) and phenotypically mutant lyc1/MO-pkd1b embryos (n=4) (5ng MO) between 56-64 hpf. PHENOTYPE:
|
|
The lyc1 mutant lymphatic phenotype is enhanced with MO-pkd1b injection and targeting calcium signaling results in a lymphatic phenotype. Related to Figure 2. (A) Quantitative real time PCR for kdrl, cdh5, prox1a, nfatc1, nrp2a, flt4 and lyve1 transcripts normalized expression at 3 dpf in sorted embryonic venous and lymphatic endothelial cells. Sorted cell populations display the predicted enrichment of marker genes. (B) Quantitative real time PCR for pkd1b transcript normalized expression against ef1a and rpl13 at 3 dpf in WT and MO-pkd1a embryos at 24hpf. pkd1b is readily detectable in whole embryo cDNA but not altered by pkd1a knockdown. (C) Quantification of parachordal lymphangioblasts in WT (n=18), lyc1 (n=9) WT/pkd1b (5ng MO) (n=21), lyc1/MO-pkd1b embryos (5ng MO) (n=23) at 56hpf. (D-E) Quantification of thoracic duct extent in (D) WT (n=48), lyc1 (n=23), WT/MO-pkd1b (5ng MO) (n=24), lyc1/MO-pkd1b embryos (5 ng MO) (n=21), and (E) WT (n=50), MO-pkd2 embryos (7.5 ng MO)(n=136) at 4dpf. (F) Quantitative real time PCR for cacna1s transcript normalized expression at 30 hpf in sorted embryonic venous and arterial endothelial cells. Endothelial expression of this calcium channel and Nifedipine target is confirmed. (G-H) The vasculature of (G) DMSO 0.05% and (H) DMSO 0.05%/Nifedipine 25 μM treated embryos in Tg(fli1a:EGFP y1; kdrl:mcherry s916). The thoracic duct is markedly absent in the presence of a calcium signaling antagonist (Nifedipine). (I) Quantification of parachordal lymphangioblasts in DMSO 0.2% (n=62) and DMSO 0.2%/Nifedipine 100μm treated embryos (n=101) at 56 hpf. PLs are unchanged in the presence of a calcium signaling antagonist (Nifedipine). (J) Thoracic duct quantification in DMSO 0.05% (n=45) and DMSO/0.05%/Nifedipine 25 µM (n=68) at 5 dpf. Thoracic duct reduction similar to lyc1 mutants is observed. (K) Quantification of thoracic duct extent in WT/MO-pkd1b/0.05% ethanol (5ng MO) (n=21), lyc1/MOpkd1b/ 0.05% ethanol (5ng MO) (n=21), MO-pkd1b/ethanol 0.05%/Bayk8644 (5ng MO) (n=28) and lyc1/MO-pkd1b /ethanol 0.05%/Bayk8644 (5ng MO)(n=15) embryos at 4dpf. A mildly penetrant lyc1 carrier was used. Remarkably, a phenotypic interaction with the calcium agonist is observed only in the mutant animals and not in the wildtype siblings. This suggests a sensitivity of mutant cells to further fluctuations in Ca2+ signalling. |
|
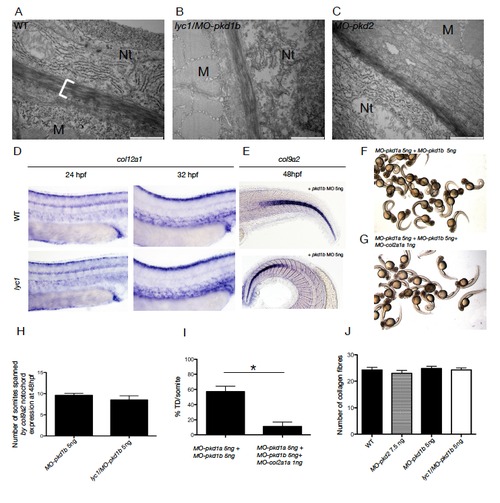
The lyc1 lymphatic vascular phenotype is independent of collagen gene expression and ECM changes. Related to Figure 2. (A-C) Electron-microscopy imaging of the peri-notochordal region in WT, lyc1/MO-pkd1b (5ng MO) and MO-pkd2 embryos (7.5 ng MO). Nt=notochord, M=muscle. (D) Expression of col12a1, a vascular collagen, was unchanged at 24 and 32 hpf in control (WT/MOpkd1b) (n=27, n=37 respectively) compared with phenotypically mutant lyc1/MO-pkd1b embryos (5ng MO) (n=37, n=12 respectively).(E) Expression of col9a2 was unchanged at 48 hpf in control (WT/MO-pkd1b) (n=10) compared with phenotypically mutant lyc1/MO-pkd1b embryos (5ng MO) (n=10). (F-G) Overall morphology of (F) MO-pkd1a/MO-pkd1b (5ng MO each) and (G) MO-pkd1a/MOpkd1b/ MO-col2a1a morphants (5,5,1 ng MO respectively). Knockdown of Col2a1a rescues the gross curvature phenotype as previously described (Mangos et al., 2010). (H). Quantification of col9a2 expression as the anterior posterior extent of expression in the notochord, delineated by somites boundaries. No increase in col9a2 extent was observed. (I) Quantification of thoracic duct extent in MO-pkd1a/MO-pkd1b embryos (n=21) and pkd1a/pkd1b/col2a1a morphants (n=19). Despite rescue of the gross curvature phenotype, TD extent is not rescued. (J) Quantification of the number of collagen fibers in the medial layer of the peri-notochordal region in WT (n=3), WT/MO-pkd2 (7.5 ng MO) (n=3), WT/MO-pkd1b (5 ng MO) (n=3) and lyc1/MO-pkd1b embryos (5ng MO)(n=3). No change was observed in peri-notochordal collagen. |
|
No evidence for a contribution of primary cilia to lymphangiogenesis. Related to Discussion section. (A-B) The vasculature Tg(lyve1:DsRed2)nz101 in (A) wild-type sibling and (B) ift88tz288 mutant embryos at 56 hpf (arrowheads indicate parachordal lymphangioblasts and white arrows indicate venous sprouts).(C-D) Overall morphology of (C) wild-type siblings and (D) ift88tz288 mutants at 5 dpf. (E-H) The vasculature Tg(lyve1:DsRed2nz101; flt1:YFPhu4624Tg) of (E,G) WT and (F,H) ift88tz288 mutants at 5 dpf. (I-J) Quantification of (I) secondary sprouts in WT (n=12) and ift88tz288 (n=10) at 56 hpf and (J) thoracic duct extent in WT (n=16) and ift88tz288 (n=18). (K-N) Overview of primary cilia localization in the trunk of (K,M) WT (n=4) and (L,N) lyc1 mutants (n=3) embryos at 30 hpf, stained for blood vessels (Kdrl-Cherry), nuclei (DAPI) and primary cilia (Acetylatedtubulin) markers. Arrowheads indicate example of discrete primary cilia. (O) An individual representative primary cilium in a lyc1 embryo. (P) Transient expression of a Pkd1-YFP BAC construct in an arterial intersegmental vessel and adjacent muscle cells at 4 dpf. DA: Dorsal Aorta, PCV: Posterior Cardinal Vein, TD: Thoracic duct |