- Title
-
The development of zebrafish tendon and ligament progenitors
- Authors
- Chen, J.W., Galloway, J.L.
- Source
- Full text @ Development
|
Expression of tendon markers during zebrafish development. At 48hpf, scxa is expressed in the (A) pharyngeal arches and (B) myosepta. (C) At 56hpf, scxa is expressed in the craniofacial region (asterisk, arrow, arrowhead). (D) Section in situ hybridization of scxa expression (arrowhead) between cartilage (c) and muscle (arrow) at 120hpf. (E-S) Expression of scxa (E-H), tnmd (I-L), xirp2a (M-P) and col1a2 (Q-S) at 72hpf. All four genes are expressed at the attachment point of the sternohyoideus muscles to the ceratohyal and basihyal cartilages (F,J,N,R, arrowhead; magnified in G,K,O,S). scxa, tnmd and col1a2 are robustly expressed in two stripes ventromedial to the palatoquadrate (F,J,R, arrow). scxa and xirp2a are expressed at the adductor mandibulae, intermandibularis and hyohyoideus muscle attachment points (F, asterisks; N). scxa, tnmd and xirp2a are also expressed at the base of the cleithrum (H,L,P, arrow). (T) Schematic ventral view of zebrafish craniofacial muscle (red), cartilage (gray) and tendon/ligament (green) populations at 72hpf. Only subsets of the muscle groups are depicted. All are ventral views of flat-mounted embryos except in B,H,L,P, which are lateral views, and in D, which is a coronal view. ac, actinotrichia; am, adductor mandibulae; bh, basihyal; c, cartilage; cl, cleithrum; ch, ceratohyal; hh, hyohyoideus; ih, interhyoideus; ima, intermandibularis anterior; imp, intermandibularis posterior; m, muscle; mc, Meckel′s cartilage; pq, palatoquadrate; sh, sternohyoideus. |
|
Tendon genes mark discrete domains joining muscle and cartilage. (A,B) scxa is expressed at points of attachment of muscle-to-cartilage and cartilage-to-cartilage in the craniofacial tissue at 60hpf. Images were generated by overlaying the bright-field and fluorescent channels. (C) scxa and tnmd are co-expressed at the base of the cleithrum (arrow). (D-F) scxa and xirp2a are co-expressed in muscle attachment points, e.g. where the interhyoideus and intermandibularis muscles intersect (D, arrowhead; enlarged in E). Single channels of confocal image are shown in E. (G-I) Colocalization of scxa and tnmd is detected between 60 and 80hpf. Expression of scxa and tnmd transcripts is temporally dynamic: there is robust expression of scxa at 60hpf followed by weaker expression after 80hpf; tnmd is weakly expressed at 60hpf and increases in expression after 80hpf. (J) At 96hpf, xirp2a and tnmd are co-expressed in regions proximal to the muscle (arrowhead). (K-M) scxa and xirp2a are co-expressed in the tail myosepta in regions medial (L) and lateral (M) to the notochord. Single channels of confocal images are shown in L,M and an asterisk marks the corresponding myoseptum in the same embryo. (N,O) At 96hpf, tnmd and col1a2 are co-expressed in regions medial to the palatoquadrate (N, arrow), at the sternohyoideus connection point (N, arrowhead), and in the myosepta (O). Ventral (A,D-J,N) and lateral (B,C,K-M,O) views of flat-mounted embryos. |
|
Expression and ultrastructural analysis of adult zebrafish craniofacial tendons and ligaments. (A,B) Coronal sections of juvenile zebrafish show tnmd expression near the sternohyoideus attachment (arrowhead) and in lateral regions attached to the mandible (arrow). tnmd is also detected in the perichondrium. Transmission electron microscopy of ligament (C,D) and tendon (F,G) longitudinal sections reveal the parallel arrangement of collagen fibrils with characteristic periodicity. Ligament (E) and tendon (H) cross-sections reveal round collagen fibrils. EXPRESSION / LABELING:
|
|
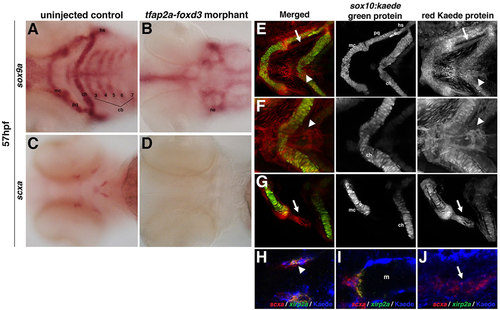
Zebrafish craniofacial tendon populations are derived from the neural crest. Morpholino-mediated knockdown of tfap2a and foxd3 results in (A,B) complete loss of sox9a-positive pharyngeal cartilage (98%, n=58) and (C,D) scxa-positive craniofacial tendon progenitors (96%, n=46) at 57hpf compared with controls. (E-G) 72hpf photoconverted sox10:kaede embryos express sox10:kaede green protein in the pharyngeal cartilage (E-G and middle panel), and the red Kaede protein from the 22hpf photoconversion is found in the two major populations of craniofacial tendon progenitors (E, arrow and arrowhead; F,G, right panel). (H-J) Colocalization of scxa and Kaede protein in photoconverted sox10:kaede embryos at 72hpf is observed in the sternohyoideus connection point (H,I; sternohyoideus muscle is labeled m) and in the ligament (J). A subset of the scxa-positive and Kaede-positive cells also co-expresses xirp2a transcripts. Arrows in E,G,J mark ligament medial to palatoquadrate; arrowheads in E,F,H mark tendon connecting the sternohyoideus to the ceratohyals. cb: ceratobranchials; ch, ceratohyal; hs, hyosymplectic; m, muscle; mc, Meckel′s cartilage; ne, neurocranium; pq, palatoquadrate. EXPRESSION / LABELING:
PHENOTYPE:
|
|
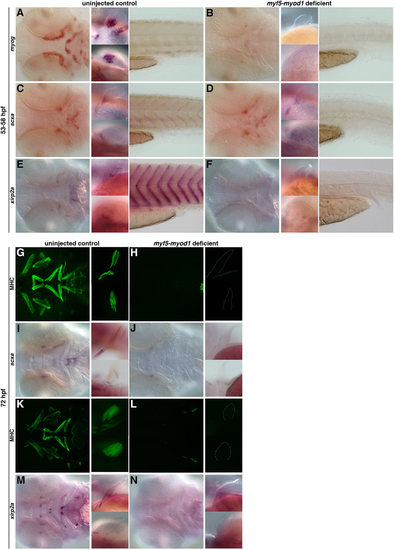
The role of muscle in the specification and maintenance of tendon populations. (A,B) Loss of myod1 and myf5 results in the complete absence of myog-positive differentiated muscles in the head (left), fin (middle) and tail (right) (93%, n=67). (C-F) In myod1-myf5-deficient embryos at 53-58hpf, scxa expression is lost in the myosepta (C,D), and xirp2a expression is completely absent in the craniofacial, pectoral fin and myoseptal tissue (E,F) compared with controls. However, loss of differentiated muscle (C,D) does not alter expression of scxa-positive tendon progenitors in the craniofacial or pectoral fin tissue (97%, n=32). (G-N) At 72hpf, myod1–/– and myf5-deficient embryos have (G,H,K,L) complete loss of myosin heavy chain (MHC) expression in the craniofacial and pectoral fin tissue and (I,J) a virtual loss of scxa expression in the head and pectoral fin tissue. (M,N) Expression of xirp2a is also missing (100%, n=19). Fluorescent images of MHC-stained flat-mounted embryos in G,H,K,L correspond to the same embryos in brightfield (I,J,M,N). EXPRESSION / LABELING:
|
|
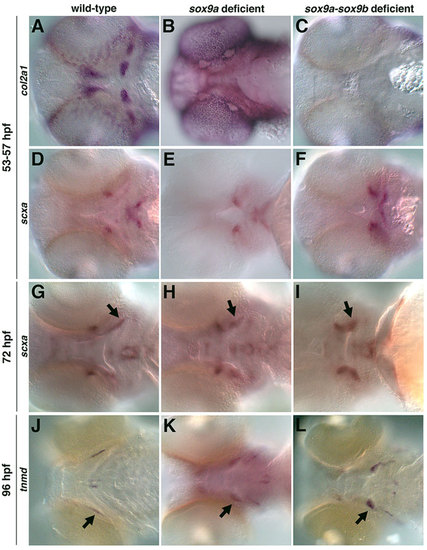
The role of cartilage in the specification and organization of tendon and ligaments. col2a1-positive pharyngeal cartilage was lost in sox9a- (B) and sox9a-sox9b-deficient embryos (88%, n=56) (C) compared with controls (A). (D-F) sox9a- and sox9a-sox9b-deficient embryos (93%, n=42) express scxa but in an altered pattern compared with controls. sox9a-sox9b-deficient embryos were identified by the loss of their otic vesicle, which only occurs in the absence of both genes. Similar expression was observed for morpholino-mediated knockdown of sox9a and sox9b together, and with sox9a or sox9b morpholino injection into crosses of sox9ahi1134 or sox9bfh313 mutants, respectively. (G,H,J,K) At 72hpf and 96hpf, sox9a- and sox9a-sox9b-deficient embryos (82%, n=57) express scxa and tnmd, but in an abnormal pattern compared with controls. The scxa- and tnmd-expressing ligaments (H,I,K,L, arrows) appear shorter and wider than controls. Expression posterior and lateral to the ligaments in K,L marks the branchiostegal rays. |
|
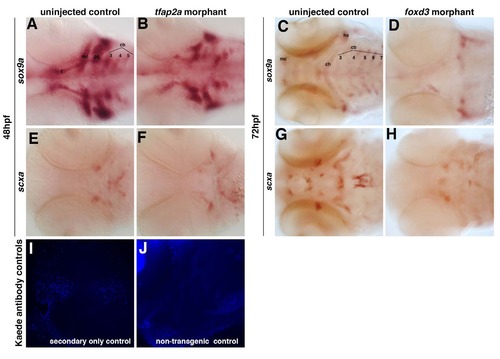
Loss of tfap2a and foxd3 individually alters the pattern but does not abolish the expression of scxa. Morpholino- mediated knockdown of tfap2a results in (A-B) reduction of sox9a-positive pharyngeal cartilage and (E-F) an altered pattern of scxa expression at 48 hpf. Morpholino-mediated knockdown of foxd3 results in (C-D) significant loss of sox9a-positive pharyngeal cartilage and (G-H) a significant loss of scxa-positive craniofacial tendon progenitors at 72 hpf. Ventral views of flat-mounted embryos. Controls for Kaede antibody staining for the secondary alone (I) or in non-transgenic embryos (J) did not show any staining when imaged at the same levels as in Figure 4 H-J. |
|
Control oligo morpholino does not affect the expression of (A-B) sox9a, (C-D) scxa, (E-F) myog, and (GH) col2a1. Injection of control scrambled oligo at comparable concentrations to that of morpholinos used does not alter expression of sox9a, scxa, myog, or col2a1 compared to controls at 60 hpf. |
|
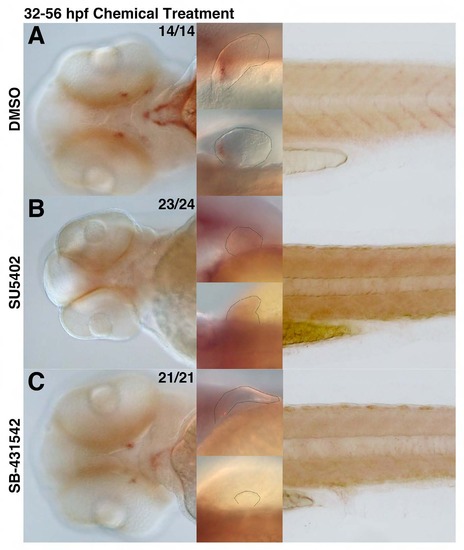
scxa-positive tendon populations are affected upon loss of FGF and TGFβ signaling. scxa expression in embryos upon incubation in chemical from 32-56 hpf. (A) DMSO-treatment with DMSO does not alter scxa expression and is used as a positive control. (B) Treatment with FGFR inhibitor (SU5402) results in complete loss of scxa-positive population in the craniofacial, pectoral fin, and myoseptal tissue. (C) Inhibition of TGFβ signaling (SB-431542) results in a significant reduction of scxa-positive population in the craniofacial, pectoral fin, and myoseptal tissue. EXPRESSION / LABELING:
|
|
Pectoral tendon populations are specified and maintained in the absence of cartilage. (A-B, D-E) sox9a morphants have loss of col2a1-positive pharyngeal cartilage and altered pattern of scxa expression at 53-57 hpf. (C, F) sox9a-sox9b deficient embryos have lost col2a1-positive pharyngeal cartilage, yet retain scxa expression at 53-57 hpf. (G-I) Embryos deficient in sox9a and/or sox9b transcripts express scxa at 72 hpf. (J-L) Embryos deficient in sox9a and/or sox9b transcripts express tnmd at 96 hpf. Representative lateral (left) and dorsal (right) views of pectoral fin tissue are indicated for each category of embryos. |