- Title
-
Protein tyrosine phosphatase PTPN9 regulates erythroid cell development through STAT3 dephosphorylation in zebrafish
- Authors
- Bu, Y., Su, F., Wang, X., Gao, H., Lei, L., Chang, N., Wu, Q., Hu, K., Zhu, X., Chang, Z., Meng, K., Xiong, J.W.
- Source
- Full text @ J. Cell Sci.
|
ptpn9a is expressed in the primitive hematopoietic sites during zebrafish embryogenesis. RNA in situ hybridization with ptpn9a probe in one-cell (A), 6-hpf (B), 15-hpf (C), 18-hpf (D), 24-hpf (E) and 36-hpf (F) embryos. Note ptpn9a expression in the posterior lateral plate mesoderm (PLPM) (C; red arrowhead) and the intermediate cell mass (ICM) (D,E; red arrowheads) regions. Lateral views are shown with anterior to the left (D–F), and dorsal views are shown with anterior to the top (C). |
|
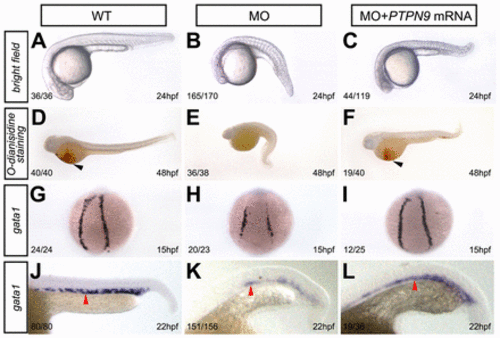
Knockdown of ptpn9a depletes embryos of primitive erythrocytes. (A–C) Brightfield images of control (A), a ptpn9a morphant (MO) (B) and a ptpn9a morphant that had been co-injected with human PTPN9 mRNA (C) at 24 hpf. (D–F) o-Dianisidine staining showed depletion of erythroid cells in ptpn9a-morphant embryos (E), compared with controls (D). Human PTPN9 mRNA partially rescued primitive erythropoiesis in ptpn9a-morphant embryos (F). In D and F, black arrowheads indicate erythrocytes. (G–L) RNA in situ hybridization was performed with gata1 probes in controls at 15 hpf (G) and 22 hpf (J), ptpn9a morphants at 15 hpf (H) and 22 hpf (K), and ptpn9a morphants that had been co-injected with human PTPN9 mRNA at 15 hpf (I) and 22 hpf (L). Note reduced gata1 expression in the PLPM (H) and ICM (K), which were partially rescued by co-injection of human PTPN9 mRNA (I,L). Red arrowheads indicate the ICM (J–L). Lateral views are shown with anterior to the left (A–C, D–F, J–L); dorsal views are shown with anterior to the top (G–I). The number of phenotypic embryos/total embryos is shown in the bottom left-hand corner. EXPRESSION / LABELING:
|
|
ptpn9a knockdown causes no defect in hematopoietic cell proliferation and apoptosis. (A,B) ptpn9a-MOs were injected into Tg(ef1α:mAG-zGem) transgenic embryos, in which green-fluorescence-positive cells are in phases S, G2 or M of the cell cycle. Note that there is little difference in the numbers of green-fluorescence-positive cells in the ICM between ptpn9a-morphant (B) and wild-type (A) embryos at 24 hpf. (C,D) The TUNEL assay showed comparable numbers of apoptotic cells in the ICM in ptpn9a morphants (D) and controls (C) at 24 hpf. (E,F) RNA in situ hybridization revealed that expression of the p53 gene was not induced in the ICM of ptpn9a-morphant embryos (F), compared with wild-type controls (E). Red arrowheads indicate the ICM. The number of phenotypic embryos/total embryos is indicated. EXPRESSION / LABELING:
|
|
Knockdown of PTPN9 interferes with erythroid cell maturation. RNA in situ hybridization was performed with βe1-globin (A–F,K,L) or βe2-globin (G–J) in controls (A,C,E,G,I,K) and ptpn9a morphants (B,D,F,H,J,L). Note the reduced βe1-globin at 26 hpf (B) and substantially diminished βe1-globin at 36 hpf (D) and at 48 hpf (F) in ptpn9a morphants, compared with controls (A,C,E). βe2-globin was almost abolished at 36 hpf (H) and 48 hpf (J) in ptpn9a morphants, compared with controls (G,I). Overexpression of human PTPN9 mRNA partially rescued βe1-globin in ptpn9a morphant embryos at 48 hpf (L), compared with controls (K). Lateral views are shown with anterior to the left (A–L). Red arrowheads indicate βe1-globin-positive or βe2-globin-positive erythroids at 36 hpf (D,H). The numbers in the bottom left-hand corners indicate the phenotypic embryos/total embryos. (M–P) Human K562 cells were infected with retroviruses containing either an empty vector (Vec) or a dominant-negative mutant PTPN9 (N9CS), or were transfected with either a scrambled construct (Scr) or PTPN9 RNAi construct (shN9). The infected or transfected K562 cells were induced with hemin for 48h. (M) The percentages of benzidine-positive cells were reduced in both N9CS and shN9 groups. Error bars represent the standard deviation, and the means of data from three independent experiments are shown. (N–P) Quantitative real-time PCR analyzed the expression of γ-globin, CD235a and CD71 in infected or transfected K562 cells after hemin induction for 48h. Relative γ-globin, CD235a and CD71 expression levels, normalized to GAPDH, were reduced in both N9CS and shN9 groups. Measurements are the means from three independent experiments±s.d., *P<0.05. |
|
Overexpressing gata1, zbp-89 or dominant-negative STAT3 rescues erythroid development in ptpn9a-morphant embryos and PTPN9-knockdown K562 cells. (A-C,G-L) βe1-globin was partially rescued in ptpn9a morphants (MO) that had been co-injected with gata1 mRNA (C), zbp-89 mRNA (I) or dominant-negative STAT3 (STAT3DN) mRNA (L), compared with wild-type (WT) controls (A,G,J) at 48 hpf. In addition, overexpression of gata1 mRNA rescued primitive erythrocytes in ptpn9a morphants (F), compared with wild-type controls (D), as revealed by o-Dianisidine staining. The numbers in the bottom left corner represent phenotypic embryos/total embryos. (M-P) Overexpression of STAT3DN rescued benzidine-positive erythroids (M) and γ-globin (N), CD235a (O) and CD71 (P) expression in K562 cells that had been infected with either mutant PTPN9 (N9CS) or transfected with PTPN9 shRNA (shN9) after induction with hemin for 48h, compared with vector (Vec) or scrambled (Scr) controls. The data represent the means of three independent experiments±s.d., *P<0.05. |
|
Ptpn9a, but not ptpn9b, is crucial for early development in zebrafish. (A) Genomic structure of ptpn9a and ptpn9b in zebrafish, showing morpholino targeting sites (ptpn9a-MO and ptpn9b-MO) and RT-PCR primers for Fig. S2H by the red lines. (B) Protein sequence alignments among human PTPN9 (hPTPN9), mouse Ptpn9 (mPtpn9), and zebrafish Ptpn9a (ptpn9a) and Ptpn9b (ptpn9b). Note hPTPN9, mPtpn9 and Ptpn9a are highly conserved while Ptpn9b has unique linker amino-acids. (C) Genomic synteny shows that Ptpn9a is a potential ortholog of hPTPN9 and mPtpn9, while Ptpn9b was derived from zebrafish genome duplication. (D-I) RNA in situ hybridization was performed with a ptpn9b probe in 1-cell (D), 6 hpf (E), 15 hpf (F), 18 hpf (G), 24 hpf (H), and 36 hpf (I) embryos. (J-K) No apparent blood defects were found in ptpn9b morphants (J, right panel), compared with wild-type controls (J, left panel). Ptpn9b-MO was injected at 10 ng per embryo. Semi-quantitative RT-PCR revealed that ptpn9b was not detectable in ptpn9b morphants (K), suggesting that ptpn9b-MO was effective. Gapdh was used as an internal control. (L-N) Co-injection with synthetic zebrafish ptpn9a mRNA rescued βe1-globin and embryo morphology in ptpn9a morphant embryos at 48 hpf (N), compared with controls (L). To prevent binding of synthetic zebrafish ptpn9a mRNA to ptpn9a-MO, we added the kozak sequence (GCCACC) before the coding district sequence (CDS) of ptpn9a. (O-T) Over-expression of either ptpn9b mRNA or truncated ptpn9a mRNA that the SEC14 domain of Ptpn9a was deleted had no rescue on βe1-globin and embryo morphology in ptpn9a morphants at 48 hpf (Q, T), compared with controls (O, R). Lateral views are shown with anterior to the left (G-J, L-T), dorsal views are shown with anterior to the top (F). |
|
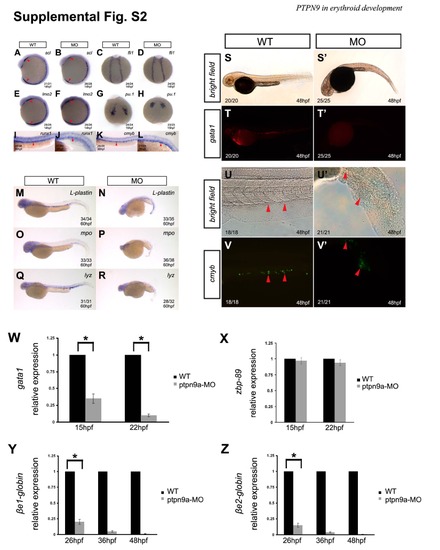
ptpn9a knockdown depletes erythrocytes and has mild effects on myeloid cells but not hematopoietic stem cells. (A-F) The hematopoietic progenitor markers scl/tal1, lmo2, and fli1 were not affected in ptpn9a morphants (B, D, F), compared with controls (A, C, E) at 14 hpf. Red arrowheads indicate the ALPM and the PLPM. (G-H) The myeloid progenitor marker pu.1 was not altered in ptpn9a morphants (H), compared with controls (G) at 15 hpf. (I-L) The hematopoietic stem cell markers runx1 and cmyb were not altered in ptpn9a morphants (J, L), compared with controls (I, K) at 36 hpf. (M-R) The myeloid genes l-plastin, myeloperoxidase (mpo) and lysozyme C (lyz) were slightly reduced in ptpn9a morphants (N, P, R), compared with controls (M, O, Q) at 60 hpf. (S-T′) Ptpn9a-MOs were injected into Tg(gata1-deRed) transgenic embryos, in which erythrocytes were labeled by DsRed. Note the erythrocytes were abolished in ptpn9a morphants at 48 hpf (T′), compared with controls (T), Bright-field images of wild-type (S) and morphant (S′) as shown in panels. (U-V′) Ptpn9a-MOs were injected into Tg(cmyb-EGFP) transgenic embryos, in which HSCs were labeled by EGFP. Note comparable numbers of EGFP-positive HSCs in the caudal hematopoietic tissue between ptpn9a morphant (V′) and wild-type (V) embryos at 48 hpf. Bright-field images of control (U) and morphant (U′). Lateral views are shown with anterior to the left (A-B; E-F; I-V′); dorsal views are shown with anterior to the top (C-D; G-H). (W-Z) Differentiated erythroid genes gata1, βe1-globin and βe2-globin reduced in ptpn9a morphants, compared with controls (W, Y and Z), but hematopoietic progenitor gene zbp-89 was normally expressed in both ptpn9a morphants and wild-type embryos (X). Erythroid gene expression was normalized by GAPDH. Measurements are the means from 3 independent experiments ± SD. *P <0.05. |