- Title
-
Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis
- Authors
- Le Guen, L., Karpanen, T., Schulte, D., Harris, N.C., Koltowska, K., Roukens, G., Bower, N.I., van Impel, A., Stacker, S.A., Achen, M.G., Schulte-Merker, S., and Hogan, B.M.
- Source
- Full text @ Development
|
Zebrafish vegfc mutants lack lymphatic vessels, but appear otherwise normal. (A-D) Confocal projections of Tg(fli1a:EGFP) show unaltered overall morphology and blood vasculature in vegfchu5055 mutants (B) compared with wild type (A). The TD (C, arrows) is absent in vegfchu5055 (D, asterisks). (E) Positional cloning linked alleles hu6124 and hu5055 to large regions of chromosome 1, containing the vegfc gene. For hu6124, 13 recombinant embryos identified at z9394 were reduced to four recombinant embryos common with z5508, identifying linkage in a large region containing vegfc. For hu5055, eight recombinant embryos at z2691 and 33 different recombinant embryos at z4694 flanked a large region of chromosome 1, also containing vegfc. (F) Sequencing of vegfc alleles. hu6410 encodes an early stop allele leading to a predicted truncated protein lacking the core (VHD) region. hu5142, hu6124 and hu5055 encode missense mutations, all in highly conserved regions of the protein (G) and predicted to be damaging by PolyPhen-2 (Adzhubei et al., 2010) with scores of 1 out of 1. (G) Alleles hu5055, hu6410, hu6124 and hu5142 all encode mutations in regions that are conserved across multiple species. (H) Alleles hu5055 and hu6410 fail to complement, with trans-heterozygote embryos displaying a lack of lymphatic structures; phenotype scored as percentage extent of thoracic duct over ten somites (total number of embryos scored n=212). (I,J) The penetrance of vegfc mutant phenotypes varies, as shown by the variable number of PLs (I) or TD extent (J). The hu6410 allele (L107X) shows the most severe defects in heterozygous and homozygous mutants, whereas hu5055 does not show haplo-insufficiency phenotypes. (K) vegfchu5055 genetically interacts with vegfr3hu4602 in trans-heterozygous embryos. Confocal projections of Tg(fli1a:EGFP; kdr-l:mCherry) showing examples of the heterogeneity observed during TD development at 5 dpf in vegfc+/-; vegfr3+/- embryos. Arrows indicate the thoracic duct, asterisks indicate absence of thoracic duct. PHENOTYPE:
|
|
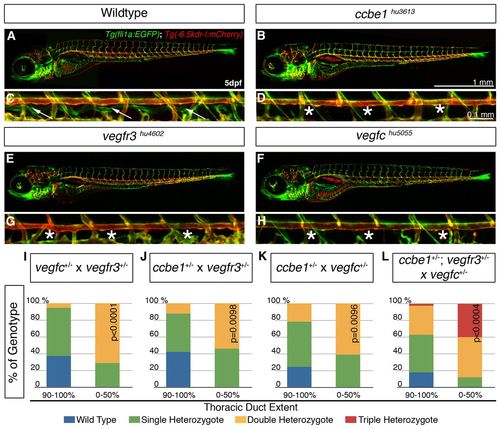
ccbe1, vegfc and vegfr3 genetically interact in double and triple heterozygous animals. (A-H) Confocal projections of Tg(fli1a:EGFP; kdr-l:mCherry) show grossly unaltered overall morphology and blood vasculature in ccbe1hu3613 (B), vegfr3hu4602 (E) and vegfchu5055 (F) mutants compared with wild type (A). The TD (C, arrows) is absent in ccbe1hu3613 (D), vegfr3 hu4602 (G) and vegfc hu5055 (H) mutants (asterisks). (I-K) ccbe1, vegfc and vegfr3 genetically interact in double heterozygote embryos, which display lymphatic defects. Offspring from vegfc+/- and vegfr3+/- carriers give rise to 28% of embryos (n=28/99) with a TD length of d50%. This population is significantly enriched (71%; n=20/28; P<0.0001) in double heterozygotes (I). Similarly in ccbe1+/- and vegfr3+/- crosses, 24% (n=24/100) embryos develop d50% of their TD, and again this population is significantly enriched (54%; n=13/24; P=0.0098) in double heterozygotes (J). In ccbe1+/- and vegfc+/- crosses, 14% (n=18/126) of embryos develop d50% of their TD, this population being significantly enriched (61%; n=11/18; P=0.0096) in double heterozygotes (K). (L) Crossing double heterozygous ccbe1+/-; vegfr3+/- animals to vegfc+/- animals, results in 21% (n=25/121) of embryos with d50% of TD: within this population, 40% (n=10/25; P=0.0004) of the embryos were triple heterozygotes, a significant enrichment from the statistical triple heterozygosity rate of 15%. PHENOTYPE:
|
|
Phenotypes driven by ectopic Vegfc/Vegfr3 signaling are suppressed in ccbe1-deficient embryos. (A) At 72 hpf, dll4 morphants display an arterial hyperbranching phenotype (arrow) driven by increased Vegfc/Vegfr3 signaling in the transgenic Tg(fli1a:EGFP) line. This phenotype was suppressed in ccbe1hu3613 mutants. Eighty-one per cent of MO-dll4 injected embryos displaying wild-type or mild phenotypes were ccbe1 mutants (n=17/21), whereas the population displaying the most severe phenotype was mainly composed of wild-type or heterozygous siblings (83%; n=139/168). (B) In dll4 morphants, arteries are sensitized to increased vegfc expression during primary sprouting. Arteries in MO-dll4, vegfc mRNA-injected embryos display aberrant, ectopic turning (arrow) as early as 30 hpf. Embryos from ccbe1 carrier incrosses, injected with 100 ng vegfc mRNA and 5 ng MO-dll4, were sorted into the phenotypic categories ‘wild type’ and ‘severe’. Genotyping revealed that 70% of the embryos displaying wild-type morphology were ccbe1 mutants (n=19/27). By contrast, the population affected by the most severe phenotype was composed of 83% wild-type or heterozygous siblings (n=122/147). (C) Confocal projections of Tg(fli1a:EGFP; flt1:tomato; hsp70l:Gal;4XUAS:vegfc) embryos show that endothelial cells in heat-shocked embryos display aberrant ectopic branching at 72 hpf. The ectopic endothelial cells are venous derived (flt1:tomato negative, arrow in Ciii). Heat-shocked embryos that were injected with 2.5 ng of MO-ccbe1 do not show this phenotype (asterisks). Scoring of the number of aberrant vISVs per heat-shocked embryo showed a significant rescue (0.12 in MO-ccbe1 injected n=22, versus 4.76 in uninjected controls n=25; P<0.0001) of the phenotype. PHENOTYPE:
|
|
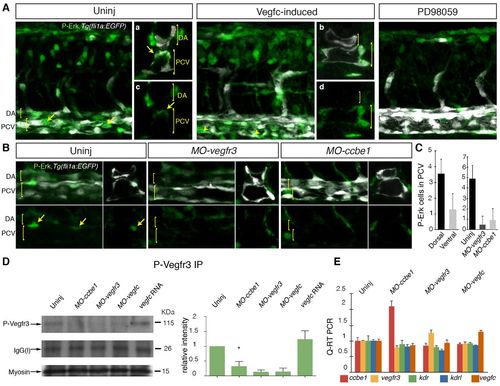
Vegfr3-dependent Erk signaling requires ccbe1 during the induction of secondary sprouting in zebrafish. (A) Analysis of phospho-Erk (P-Erk) expression in 32 hpf embryos. P-Erk (green) and fli1a:EGFP (white) images (lateral view) show P-Erk detected broadly in whole-mount and cross-sectioned (right-hand panels, merge upper, P-Erk lower) control embryos. Signal was increased in Vegfc-induced (dll4 MO + vegfc mRNA-injected) embryos in the posterior cardinal vein (n=8/8; Vegfc-induced embryos all showed ectopic expression in the ventral wall of the PCV). Cross section merged channel images shown in a and b, P-Erk only in c and d. Treatment with the Erk inhibitor PD98059 led to a reduction in all P-Erk staining. Arrows indicate P-Erk expression in the dorsal PCV. DA (dorsal aorta) and PCV (posterior cardinal vein) are indicated. (B) Comparison of P-Erk staining in control uninjected (left), MO-vegfr3 and MO-ccbe1 embryos. Upper panels are merged images and lower P-Erk only, viewed laterally (left) and cross-sectioned (right). Cross sections (right) are from separate embryos. Arrows indicate P-Erk expression in the dorsal PCV. DA and PCV are indicated. (C) Quantification of P-Erk-positive cells in the cardinal vein located in the dorsal compared with ventral wall (left-hand graph). Scores through individual sections of z-stack images from 12 control embryos, scored laterally across three somites in the trunk. Quantification of P-Erk-positive cells in the cardinal vein in control and MO-injected conditions (right-hand graph) (scores from n=10 control embryos, n=13 MO-vegfr3-injected and n=15 MO-ccbe1-injected embryos). (D) Immunoprecipitation (IP) and western blot (IB) detection of phosphorylated Vegfr3 at 32 hpf in wild type and in ccbe1, vegfr3, vegfc morphant and vegfc mRNA-injected embryos. The level of phosphorylated Vegfr3 is markedly reduced in ccbe1, vegfr3 and vegfc morphants, but is increased in vegfc-mRNA injected (500 ng) embryos compared with wild type (D, upper blot, IP for phospho-Vegfr3 and IB detection with phospho-Vegfr3). Loading controls were: the IgG light chain [IgG(l)] present in all blots after IP (D, middle blot), and Myosin to monitor protein input in IPs (D, lower blot). Quantification of Vegfr3 phosphorylation (relative to the loading control) based on three independent experiments is shown in right-hand panel. The decrease in MO-ccbe1 compared with uninjected controls is statistically significant (P<0.05). (E) qPCR analysis of the expression of ccbe1, vegfr3, vegfc, kdr and kdrl in uninjected control and MO-ccbe1-, MO-vegfc-, and MO-vegfr3-injected embryos. Error bars represent s.d. (C) or s.e.m. (D,E). |
|
Ccbe1 enhances Vegfc-driven sprouting and regulates levels of bioactive VEGFC in vitro. (A) Confocal projections at 32 hpf of Tg(shh:ccbe1), Tg(shh:vegfc) and Tg(shh:ccbe1;shh:vegfc) in a Tg(fli1a:EGFP) background. Co-overexpression of ccbe1 and vegfc in the floorplate leads to aberrant ectopic turning of the ISVs at 32 hpf (upper panels; n=32/36; P<0.0001). At 48 hpf, ccbe1 overexpression in the floorplate does not result in any phenotype, whereas vegfc-overexpressing animals display hyperbranching of the ISVs, and enhanced endothelial cell accumulation at the horizontal myoseptum (arrowhead). ccbe1 and vegfc co-overexpression in the floorplate also leads to hyperbranching ISVs, and to a marked accumulation of endothelial cells at dorsal aspects of the embryo (arrow). (B) Western blot of 293EBNA-1 cells (stably expressing VEGFC) indicate that CCBE1 is detected in the lysate of cells transfected with CCBE1 plasmid, but not in controls. (C) An increase in the levels of all forms of VEGFC is detected in the medium of CCBE1-transfected cells, compared with control cells. The mature form of VEGFC (detected at <23 kDa) is predominant. (C2) Relative intensity (split axis) of the different processed forms of VEGFC presented in C based on multiple exposures. Note the saturation of the mature form in C. (D) qPCR showing that CCBE1 transfection does not affect VEGFC mRNA levels in vitro in 293EBNA-1 cells stably expressing VEGF-C (D, left panel). Consistent with this, in zebrafish embryos the injection of vegfc or ccbe1 mRNA does not affect the endogenous levels of the other (D, right panel). Error bars represent s.e.m. |
|
Ectopic expression of mature VEGFC rescues secondary sprouting in ccbe1 morphants. (A) Confocal projections of Tg(fli1a:EGFP) at 54 hpf. Knock down of ccbe1 or vegfr3 leads to a loss of PLs at the horizontal myoseptum (arrowheads and asterisks). Ectopic expression of the mature form of VEGFC strongly rescues PL formation in ccbe1 morphants but not in vegfr3 morphants. Arrows indicate hyperbranched ISVs. (B) Quantification of PL formation at 54 hpf. In wild type, 98% (n=54/55) of embryos develop PLs, whereas in MO-ccbe1-injected embryos <4% (n=2/52) do. PL development is rescued to 74% (n=29/39) in ccbe1 morphants transiently overexpressing ΔNΔCVEGFC (P<0.0001). This rescue was never observed in vegfr3 morphants with all embryos devoid of PLs (n=23/23). (C) Quantification of ISV hypersprouting at 54 hpf. ISV hypersprouting was observed in wild-type embryos (93%; n=40/43), with mild reductions in ccbe1 morphants (79%; n=31/39) and vegfr3 morphants (65%; n=15/23) after ΔNΔCVEGFC overexpression. PHENOTYPE:
|
|
Morphology of vegfc, ccbe1 and vegfr3 mutants and additional phenotypes of vegfc mutants. (A-D) Mutant hu5055 that specifically lacks lymphatic vasculature (Figure 2F and 2H) (D) is undistinguishable from vegfr3 (C) and ccbe1 (B) mutants. (E-H) Gross morphology of vegfc mutants. Confocal projections in the Tg(fli1a:EGFP) background. At 5 dpf, vegfchu6124 homozygote mutant display a specific loss of the lymphatic vasculature with apparently normal blood vasculature (F), as compared to wildtype (E). Mutant embryos that survive after 5dpf, display severe edema in the trunk area, as well as around the eyes (H) compared with wildtype siblings (G). (I-K) Phenotypes of heterozygous and homozygous vegfc mutants. (I) Angiographies in Tg(fli1a:EGFP) transgenic wildtype sibling, vegfchu6410 heterozygote and homozygote mutant embryos reveal the presence of blood flow and thoracic duct (TD) defects in mutants. In the vegfchu6410 early stop allele, heterozygote mutant embryos display a partial loss of TD development while in homozygotes no TD is observed. (J) Secondary sprouting in plcg1 morphants. In plcg morphants, arteries are missing facilitating the visualization of venous sprouts. As compared to wildtype, only a few secondary sprouts are observed in heterozygote mutants, and even fewer in homozygote mutant embryos. (K) PLs in a wildtype sibling, vegfchu6410 heterozygote and homozygote mutant embryos. As compared to wildtype, heterozygote embryos develop less PLs. In homozygote vegfchu6410 mutants no PLs are present. PHENOTYPE:
|
|
Additional controls for vegfc RNA and MO-dll4 injections in wildtype and ccbe1 mutants. In wildtype embryos as well as in ccbe1 mutants, the single injection of MO-dll4 produces no phenotype at 30 hpf. As previously reported (Hogan et al, 2009) vegfc RNA injections in wildtype produce only a weak phenotype in the ISVs, that is not observed in ccbe1 mutants. |
|
Validation of the Tg(UAS:vegfc), Tg(shh:ccbe1) and Tg(shh:vegfc) transgenic line. (A) The Tg(4xUAS:vegfc) line, was crossed to the Tg(hsp70l:Gal4)(Scheer et al, 2001) to provide inducible ubiquitous expression of vegfc after heat-shock, or to the Tg(s1173:Gal4)(Scott & Baier, 2009) to provide specific expression in the tectal cell bodies, hindbrain, strong ocular muscles, and eye: bipolar cells. Schematic representation of the transgenic constructs. (B) In situ hybridization (as described (Schulte-Merker, 2002)) at 24hpf in a clutch of incrossed Tg(hsp70l:Gal4;4xUAS:vegfc) embryos. n=32/83 embryos displayed ectopic vegfc expression with an expected frequency of 50%. In a clutch of incrossed Tg(s1173:Gal4;4x- UAS:vegfc), 22/68 embryos displayed ectopic vegfc expression with an expected frequency of 50%. (C) Schematic representation of the shh:ccbe1 and shh:vegfc contructs. We generated these constructs using the MiniTol2 vector(Balciunas et al, 2006) to specifically overexpress either of these genes in the FP using the shh promoter and a FP specific enhancer. The effective expression of these genes can be monitored due to the integration of an IRES tagRFP or IRES mturquoise reporter. (D) Confocal projections in the Tg(fli1a:EGFP) background. At 56hpf in Tg(shh:ccbe1) embryos, RFP fluorescence is detected in the floorplate (FP). |
|
Tg(shh:vegfc) partly rescues lymphatic development in ccbe1hu3613 mutants but not in ccbe1 morphants. (A) Confocal projections in the Tg(flt4:YFP) background. In wildtype embryos PLs are visible at 3.5dpf (arrows) and TD is present at 5dpf (arrow heads), but not in ccbe1hu3613 mutants, PLs and TD are missing (asterisks). However upon vegfc overexpression in the Tg(shh:vegfc) some PLs (arrows) and TD fragments (arrow heads) are observed in ccbe1hu3613 mutants. (B) Quantitation of PLs and TD rescue in the Tg(shh:vegfc) in the absence of ccbe1. When vegfc is ectopically expressed in the floorplate, more than 70% of ccbe1hu3613 mutants have PLs, and 55% develop TD fragments. (C) Confocal projections in the Tg(flt4:YFP) background. In wildtype embryos PLs are visible at 3.5dpf (arrows), but not in ccbe1 morphants (asterisks). Upon vegfc overexpression in the Tg(shh:vegfc) PLs (asterisks) are not rescued in ccbe1 morphants. (D) Quantitation of PLs rescue in the Tg(shh:vegfc) in wildtypes and ccbe1 morphants. When vegfc is ectopically expressed in the floorplate, 0% of ccbe1 morphants have PLs. (E) In the Tg(hsp70l:Gal4;4xUAS:vegfc) line no PL or TD rescue was observed after ccbe1 morpholino injection. |