- Title
-
Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction
- Authors
- Charvet, B., Guiraud, A., Malbouyres, M., Zwolanek, D., Guillon, E., Bretaud, S., Monnot, C., Schulze, J., Bader, H.L., Allard, B., Koch, M., and Ruggiero, F.
- Source
- Full text @ Development
|
Expression and distribution of zebrafish col22a1 mRNA and protein. (A) RT-PCR analysis of zebrafish col22a1 at different developmental stages. β-Actin was used as a loading control. (B) Whole-mount in situ hybridization with col22a1 antisense probe in the trunk of 24 hpf (a), 48 hpf (b) and 72 hpf (c) embryos. Lateral view of a 72 hpf whole embryo (d) and zoomed image of a somite boundary (e). (a-e) Lateral views with anterior to the left; (a-c) somites in the posterior region of yolk extension are shown. Scale bars: 50 μm in a-c; 200 μm in d; 10 μm in e. (C) Western blot with anti-COLXXII of protein extracts from animals at different stages. COLXII and myosin heavy chain antibodies were used as collagen and late muscle differentiation marker controls, respectively. Antibodies to GAPDH and acetylated tubulin were used as loading controls. Number on the left indicate sizes (in kDa) of protein standard markers. hpf, hour post-fertilization. (D) Lateral views of whole-mount immunofluorescence staining with anti-COLXXII of 26 hpf (a) and 48 hpf (b) embryos. (a) Arrows indicate COLXXII staining that concentrated along myosepta. (c-e) Double immunostaining of a 72 hpf embryo with anti-COLXXII (c, red), dystrophin (d, green) and merge (e). Anterior is towards the left. Scale bars: 50 μm in a; 150 μm in b; 20 μm in c-e. |
|
Morpholino knockdown of col22a1 in developing zebrafish. (A,B) Efficacy, specificity and functional duration of col22a1 morpholino-knockdown in developing embryos. (A) Western blots of MO22b-injected (MO) and uninjected (WT) embryo protein extracts with antibodies to COLXXII, myoseptal marker COLXII or myosin heavy chain. Loading controls are GAPDH and acetylated tubulin. Data from one representative experiment are shown. Histogram shows densitometric quantification of relative COLXXII and COLXII expression levels normalized to acetylated tubulin. Data are mean±s.e.m. (n=3), *P<0.05, **P<0.01. (B) Immunofluorescence with antibodies to COLXXII of 48 hpf embryos injected with MO22a, MO22b and five-base mismatch morpholino (MS22). Scale bars: 20 μm. (C,D) Light microscopy analysis of the phenotype. (C) Representative images of 48 hpf uninjected embryos, embryos injected with control MS22, and moderate and severe phenotypes of col22a1 morphants (MO22b). Histogram shows percentage of MO22-injected larvae displaying no, moderate or severe phenotypes at different developmental stages (n=250; number of injections=3). (D) U-shaped somites of 96 hpf MO-injected larvae (MO22b) compared with the V-shape somites of uninjected larvae (arrows). Scale bars: 20 μm. (E) Birefringence of 4 dpf MO22-injected larvae. Representative images of larvae injected with morpholinos against col22a1 (MO22, moderate phenotype), lama2 (MO-lama2) and itga7 (MO-itga7) compared with uninjected larvae. Quantification of birefringence measurements. Values represent mean birefringence normalized to uninjected larvae. Anterior is towards the left. |
|
col22a1 knockdown leads to muscular dystrophic phenotype. (A) Recording of contraction in response to single supramaximal electric shocks applied to wild-type and MO-22-injected larvae. Twitch responses (middle panel) and mean contraction amplitudes (bottom panel) of 5 dpf wild-type (black) and MO22-injected (gray) larvae. Data are mean±s.e.m. ***P<0.001. (B) 96 hpf uninjected (WT) and MO22-injected (MO22) larvae. Light microscopy (top panel) revealed muscle detachment and retraction (MO22, arrows) from vertical myoseptum. Acridine Orange staining (middle panels): asterisks point to Acridine Orange-positive retracted muscle fibers in MO22 and arrows indicate myoseptum. Histology (bottom panels) of MO22 shows cell-free spaces, reduced MTJ interdigitations (arrows) and clear appearance of myosepta compared with WT. Asterisk indicates a detached and retracting muscle fiber. (C) Western blots with antibodies to phospho-Akt (P-Akt) and total Akt protein (Akt) or phospho-ERK1/2 (P-ERK1/2) and total ERK protein (ERK1/2) of extracts from uninjected (wt) and tricaine-treated (Tri) wild-type and MO22-injected (MO) embryos at different stages. Loading controls are antibodies to COLXII and acetylated tubulin. Data from one representative experiment are shown. Only one band is detected with ERK1/2 antibodies, as reported for zebrafish (Ng et al., 2012). Histograms show densitometric quantification. Phospho-protein levels were normalized against total protein levels. Data are mean±s.e.m. (n=3). |
|
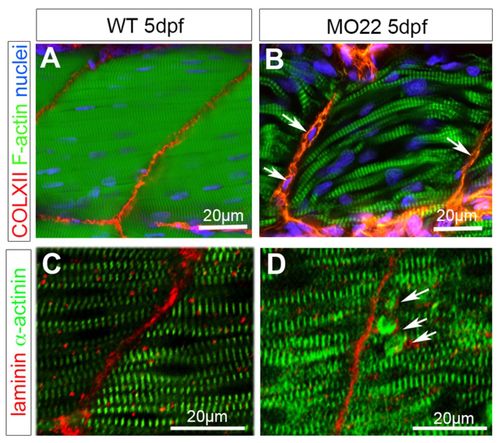
TEM reveals defects in MTJ and myosepta ultrastucture, and identifies the rupture site at the outer surface of the basement membrane. Micrographs of 5 dpf uninjected (A,C,E) and MO22-injected (B,D,F-H) larvae. (A,B) Sarcomeric organization of skeletal muscle in morphants (B) and uninjected larvae (A). (C,D) Detail of the sarcolemma and basement membrane structure (arrows) in wild type (C) and morphants (D). (E-H) Detail of MTJ and myoseptum structure in wild type (E) and morphants (F-H). Asterisks show a gap corresponding to detached and retracted fiber (F) and tears between myoseptum and muscle fibers (G,H). At rupture sites, basement membrane remains attached to sarcolemma (G,H, arrows) and is torn away with fiber detachment and retraction (F, arrow). Contrary to control (E), fibroblasts (fb) are observed in damaged myosepta (F-H). ms, myosepta; mf, muscle fiber. PHENOTYPE:
|
|
TEM reveals defects in MTJ and myosepta ultrastucture, and identifies the rupture site at the outer surface of the basement membrane. Micrographs of 5 dpf uninjected (A,C,E) and MO22-injected (B,D,F-H) larvae. (A,B) Sarcomeric organization of skeletal muscle in morphants (B) and uninjected larvae (A). (C,D) Detail of the sarcolemma and basement membrane structure (arrows) in wild type (C) and morphants (D). (E-H) Detail of MTJ and myoseptum structure in wild type (E) and morphants (F-H). Asterisks show a gap corresponding to detached and retracted fiber (F) and tears between myoseptum and muscle fibers (G,H). At rupture sites, basement membrane remains attached to sarcolemma (G,H, arrows) and is torn away with fiber detachment and retraction (F, arrow). Contrary to control (E), fibroblasts (fb) are observed in damaged myosepta (F-H). ms, myosepta; mf, muscle fiber. |
|
Genetic interaction between col22a1 and itga7. Synergistic genetic interactions between zebrafish col22a1 (COLXXII) and itga7 (integrin α7β1) (A), lama2 (laminin α2) (B) or dag1 (dystroglycan) (C), and between lama2 and itga7 or dag1 (D), and mean frequencies of muscle detachment phenotype observed in morphants. Images show lateral views of 72 hpf embryo tail musculature stained with phalloidin-rhodamine after injection, alone or in combination, of sub-phenotypic doses of morpholinos against col22a1, itga7 (A), lama2 (B) and dag1 (C). Scale bars: 50 μm. Data are mean±s.e.m. (n=3). ***P<0.001; **P<0.01. PHENOTYPE:
|
|
Protein rescue of MO22-injected embryos. (A-F) 72 hpf and (G-I) 5 dpf uninjected (WT) and embryos injected with 0.16 ng hCOLXXII protein, with 0.5 pmol MO22 or in combination (MO22+hCOLXXII). (A-F) Whole-mount fluorescence staining with phalloidin-rhodamine of embryos stimulated (w/stim, D-F) or not (wo/stim, A-C) with high-frequency field. (G-I) TEM micrographs of skeletal muscle at 5 dpf. Scale bars: 50 μm in A-F; 2 μm in G-I. |
|
A. Generation of rabbit antibodies against the zebrafish VWA domain. Lane 1, 10% SDS-PAGE analysis of the purified recombinant VWA domain produced in 293-HEK cells - The band migrates at the expected position. Lane 2, Western-blot analysis of 293-HEK conditioned media using the polyclonal antibodies obtained after rabbit immunization with the recombinant VWA domain (anti-COLXXII). B. Western-blot analysis of recombinant fulllength zebrafish collagen XXII incubated with (+) or without (-) collagenase as indicated. Zebrafish col22a1 cDNA was subcloned into the XhoI-XbaI sites of the pCS2+ vector (Turner and Weintraub, 1994) and the construct was used to transiently transfect 293-HEK cells. In absence of collagenase, COLXXII antibodies recognize the zebrafish full-length recombinant COLXXII protein as a band migrating at the expected molecular mass of the protein (200 kDa, lane 1). The collagenase digestion product revealed a 70 kDa fragment that corresponds to the undigested non-collagenous N-terminal domains, VWA plus TSPN (lane 2). A, B: protein standard size markers on the left. C-D. Immunofluorescence analysis of collagen XXII expression in adult zebrafish. Frozen cross section (C) and lateral section (D) stained with anti-COLXXII. Bars = 20 μm. E-F. Whole mount confocal immunofluorescence staining of 5dpf wild type larvae with anti-COLXXII (E) or anti-COLXII (F), actin is detected with phalloidin-rhodamine (red) and nuclei are stained with Hoechst-33344 (blue). |