- Title
-
Differential roles for 3-OSTs in the regulation of cilia length and motility
- Authors
- Neugebauer, J.M., Cadwallader, A.B., Amack, J.D., Bisgrove, B.W., and Yost, H.J.
- Source
- Full text @ Development
|
3-OST-5 is required in whole embryos and cell autonomously in Kupffer’s vesicle for LR development. (A,B) Differential interference contrast images of 48 hpf embryos. Uninjected (A) and 3-OST-5 MO-injected (B) embryos at 48 hpf showing dose-dependent curly down tail phenotype. Both 3-OST-5 MOs showed a dose-dependent increase in both heart and gut reversals. (C,D) cmlc2-gfp transgenic fish (expressing GFP in cardiomyocytes) were used to visualize normal left looping (C) and reversed right looping (D) in 3-OST-5 MO1 embryos at 48 hpf. (E) Percentages of reversed heart and gut looping in 3-OST-5 morphants. Injection of 3-OST-5 MO1 (n=120; **P<0.001) or 3-OST-5 MO2 (n=121; **P<0.001) resulted in significant heart and gut looping compared with uninjected embryos (n=361). Co-injection of 3-OST-5 MO1 and 3-OST-5 ‘rescue’ mRNA resulted in a significant rescue of heart and gut orientation (n=137; **P<0.001). Injection of 3-OST-5 ‘rescue’ mRNA alone (n=171) or 3-OST-3Z MO (n=106) did not alter normal heart and gut looping. Error bars represent s.d. for all heart and gut looping treatments. (F) Representative images of normal (left-sided), reversed (right-sided), absent and bilateral spaw expression in 3-OST-5 morphants. Percentages of normal (left-sided), reversed (right-sided), absent, and bilateral spaw expression in the LPM of 3-OST-5 morphants (MO1, n=183; MO2, n=206), 3-OST-3Z morphants (n=240) or uninjected control embryos (n=220). 3-OST-5 morphants had a preponderance of bilateral expression, in comparison with predominantly left-sided expression in uninjected and 3-OST-3Z MO1-injected embryos. Embryos injected for DFC/KV-targeted knockdown, DFC3-OST-5 MO1 (n=112), showed a high rate of bilateral spaw expression. This is in contrast to DFCcontrol MO (n=97), Yolk3-OST-5 MO1 (n=61) and Yolkcontrol MO (n=90) injected embryos showing left-sided spaw expression. |
|
3-OST-5 is required for KV cilia length. (A,B) KV in uninjected (A) and 3-OST-5 MO1-injected (B) embryos at 8 SS. Cilia were labeled with anti-acetylated tubulin (green) and cells labeled by a marker of polarized epithelia anti-aPKC (red). (C) Quantification of cilia length. 3-OST-5 MO1 injected embryos (1429 cilia, 29 embryos) had shorter cilia at 8 SS compared with uninjected control embryos (1379 cilia, 27 embryos; *P<6.19e-10). (D,E) Beads tracks showing KV morphology and bead flow. 3-OST-5 MO1 morphants (E) lacked the coordinated movement seen in uninjected embryos (D) or 3-OST-3Z morphants (supplementary material Movies 1 and 2). Each color represents a single tracked bead. (F) Average velocity of fluorescent beads was significantly slower in 3-OST-5 MO1 morphants (five embryos, five beads/embryo; *P<0.001) compared with either uninjected (eight embryos, five beads/embryo) or 3-OST-3Z MO1 (three embryos, five beads/embryo) controls. Error bars represent s.e.m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
3-OST-5 function is required for FGF signaling to regulate cilia length. (A,B) Motor molecule dnah9 mRNA was equivalently expressed in the DFC (white arrows) of tailbud stage in uninjected embryos (A; n=99) and 3-OST-5 MO1-injected embryos (B; n=62). (C,D) Ciliogenic transcription factor gene foxj1a expression in uninjected tailbud stage embryos (C, white arrow; n=111) was diminished or absent in 36% of 3-OST-5 MO1-injected embryos (D, red arrow; n=75). (E,F) Similarly, ciliogenic transcription factor gene rfx2 expression in uninjected 90% epiboly embryos (E, white circle; n=175) was diminished in 3-OST-5 MO1 DFCs (F, red circle; n=92). (G,H) FGF pathway response gene sef expression in DFCs of 90% epiboly uninjected embryos (G, white arrow; n=85) was diminished in 3-OST-5 MO1-injected embryos (H, red arrow; n=44). See Fig. 5 for quantification of in situ hybridization. (I) Schematic of experiments to test interactions between FGF signaling and 3-OST-5 function. Clutches of embryos from heterozygous crosses of fgf8 hypomorphic mutants were divided into two groups: uninjected or injected with sub-threshold dose of 3-OST-5 MO embryos. At 10 SS, individual embryos were fixed, assayed for KV cilia length, and then individually genotyped for fgf8/ace alleles by HRMA. (J) Average cilia length in WT, heterozygous and homozygous fgf8/ace mutants that were not injected or had been injected with a sub-threshold dose of 3-OST-5 MO1. Homozygous fgf8 mutant embryos injected with sub-threshold dose of 3-OST-5 MO1 had statistically shorter cilia length (**P<0.001) compared with all other classes of embryos. Error bars represent s.e.m. (K) Schematic summarizing 3-OST-5 modulation of FGF signaling and ciliogenic transcription factors to control cilia length. EXPRESSION / LABELING:
PHENOTYPE:
|
|
3-OST-6 cell-autonomously controls KV cilia motility and LR patterning, but not cilia length. (A) 3-OST-6 morphant at 48 hpf. Except for heart and gut anomalies, 3-OST-6 morphants were indistinguishable from WT embryos at the gross level. (B) Percentages of reversed heart and gut looping in 3-OST-6 morphants (MO1: n=172, **P<0.001; MO2: n=89, **P<0.001) were increased compared with uninjected embryos (n=411). Co-injection of 3-OST-6 MO1 and MO-resistant 3-OST-6ΔTM ‘rescue’ mRNA resulted in a significant rescue of heart and gut orientation (n=184; **P<0.001). Overexpression of 3-OST-6ΔTM mRNA alone did not alter normal heart and gut looping (n=140). Error bars represent s.d. (C) Percentages of left-sided, right-sided, absent, and bilateral spaw in 3-OST-6 morphants (MO1, n=159; MO2, n=165; uninjected, n=220). DFC3-OST-6 MO1 embryos (n=150) showed a high level of bilateral spaw expression, statistically distinct (**P<0.001) from controls: DFCControl MO (n=145), Yolk3-OST-6 MO1 (n=79), and YolkControl MO (n=90). (D) 3-OST-6 morphants had normal KV cell differentiation (red) and normal cilia length (green) that was comparable to control embryos (see Fig. 2A). (E) 3-OST-6 MO (920 cilia, 18 embryos, 8 SS) had cilia lengths comparable to uninjected embryos (P<0.85). Error bars represent s.e.m. (F) 3-OST-6 MO1 morphants lacked directed movement of beads injected into KV. Each color represents a single tracked bead. (G) Average velocity of fluorescent beads injected into KV. Flow velocity was significantly decreased in 3-OST-6 MO1 (five embryos, five beads/embryo; **P<0.001) compared with controls (see Fig. 2D; supplementary material Movies 1 and 2). Error bars represent s.d. (H) Radius of bead movement assay. Concentric circles were placed at bead start. Beads were scored as exiting the inner circle with a radius of 12.5 µm and/or outer circle with a radius of 25 µm. Each color represents a single tracked bead. (I) Quantification of the radius of bead movement. EXPRESSION / LABELING:
PHENOTYPE:
|
|
3-OST-6 regulates distinct cell signaling pathways to control cilia motility. (A-D) In contrast to diminished expression in 3-OST-5 MO-injected embryos (Fig. 3), 3-OST-6 MO1-injected embryos had normal expression of ciliogenic transcription factors rfx2 (A; n=74) and foxj1a (B; n=84). Motor molecule dnah9 (C; n=60) and the FGF-response gene sef (D; n=39) were also normally expressed in 3-OST-6 MO1-injected embryos. (E-G) Motor molecule kif3b mRNA normally expressed in the DFCs of 90% epiboly uninjected embryos (E, white arrow; n=79) and 3-OST-5 MO1-injected embryos (F, white arrow; n=48) was diminished in 3-OST-6 MO1-injected embryos (G, red arrow; n=38). (H) Percentages of embryos with normal levels of DFC expression of each marker gene in WT uninjected (green), 3-OST-5 MO1-injected (yellow) and 3-OST-6 MO1-injected (blue) embryos. *P<0.05, **P<0.001. The data represent a compilation of at least three independent experiments. (I-K) Electron micrographs showing cross-sections of cilia in the KV. Inner (blue arrows) and outer (blue arrowheads) dynein arms were visible in WT (I; n=3 embryos) and 3-OST-5 MO1-injected embryos embryos (J; n=4 embryos; P<1 compared with WT). By contrast, inner (red arrow) and outer (red arrowhead) dynein arms were largely absent in 3-OST-6 morphants (K; n=4 embryos; P<0.0018 compared with 3-OST-5 MO1 and WT). EXPRESSION / LABELING:
PHENOTYPE:
|
|
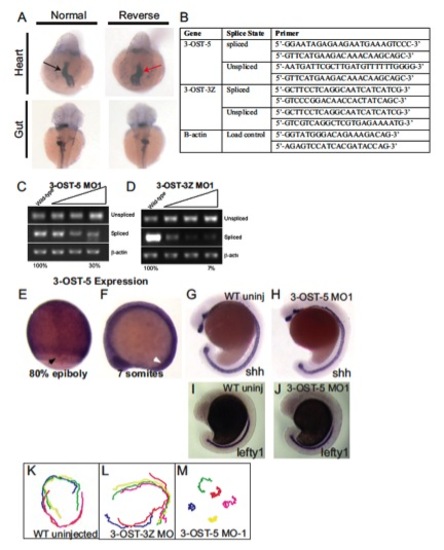
Splice-blocking efficacy, primers used to assay splicing, left-right markers examined in 3-OST-5 morphants, tracks of fluorescent beads injected into the KV at 6 SS, 3-OST-5 RNA expression, midline marker expression. (A) Representative in situ images illustrating normal (black arrows) and reversed (red arrows) heart (cmlc2) and gut (fkd2) looping in 3-OST-5 morphants. (B) Table containing primers used for detecting spliced versus unspliced transcript when embryos were injected with translational splice blocking morpholinos. (C) Dose-dependent splice blocking efficacy of 3-OST-5 MO1. Increasing amounts of MO injected resulted in a 70±12% decrease of spliced mRNA. (D) Dose-dependent splice blocking efficacy of control 3-OST-3Z MO1. Increasing MO dose resulted in a 93±4% decrease of spliced mRNA. ImageQuant TL software was used to semi-quantitatively measure band intensity. The values listed are the percentage of spliced product compared with uninjected WT. (E,F) Whole-mount in situ analysis of 3-OST-5 mRNA, which was expressed ubiquitously throughout the embryo during epiboly (E) and early somitogenesis (F). Black arrow indicates staining in DFCs, white arrow indicates the position of KV. (G,H) Whole-mount in situ analysis of shh expression in uninjected (G) and 3-OST-5 morphants (H). (I,J) Whole-mount in situ analysis of lefty1 expression in the midline of uninjected (I, n=113) and 3-OST-5 morphants (J, n=82), 3-OST-5 morphants had normal expression of lefty1 expression in the midline. (K-M) Tracks of fluorescent beads injected into KV of uninjected (K) and 3-OST-3Z MO1 embryos (L) with normal bead flow, and 3-OST-5 MO1 embryos (M), displaying uncoordinated bead movement due to short but motile cilia. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Splice-blocking efficacy, primers used to assay splicing, tracks of fluorescent beads injected into the KV at 6 SS, 3-OST-6 RNA expression, midline marker expression. (A) Analysis of 3-OST-6 MO2 splice-blocking efficacy. Increasing amounts of MO injected resulted in an 80±20% decrease of spliced mRNA. Analysis was performed as described in Fig. S1. (B) Table containing primers used for detecting spliced versus unspliced transcript when embryos were injected with translational splice blocking morpholinos. (C,D) Whole mount in situ analysis of 3-OST-6 mRNA, which was expressed ubiquitously throughout the embryo during epiboly (C) and early somitogenesis (D). Black arrow indicates staining in DFCs, white arrow indicates the position of KV. (E) Whole-mount in situ analysis of shh expression, which was normal in 3-OST-6 morphants (n=109). (F) Whole-mount in situ analysis of lefty1 expression in the midline, was normal in 3-OST6 morphants (n=49). (G) Bead tracks of fluorescent beads injected into 3-OST-6 MO1 embryos showing a lack of bead movement, indicative of nonmotile cilia. EXPRESSION / LABELING:
|