- Title
-
Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Authors
- Anderson, R.M., Delous, M., Bosch, J.A., Ye, L., Robertson, M.A., Hesselson, D., and Stainier, D.Y.
- Source
- Full text @ PLoS Genet.
|
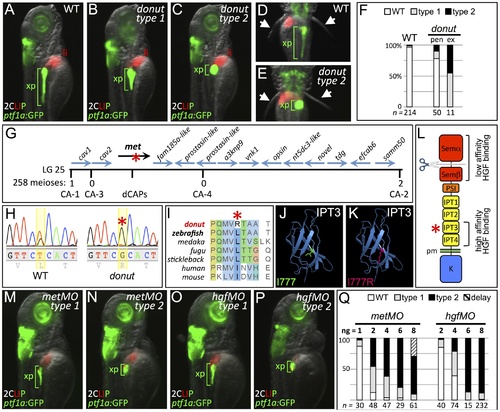
donuts908 is a hypomorphic allele of met. (A–E) Exocrine pancreas (xp) structure marked by ptf1a:GFP expression in 3 dpf WT (A,D), and in type 1 (B) and type 2 (C,E) donut mutants. The pectoral fins (arrowheads) of donut mutants lack muscle tone, often asymmetrically, leading to their “open wing” appearance (right arrow, E). (F) Penetrance (pen) and expressivity (ex) of the donut phenotype. A small fraction of WT embryos shows donut-like pancreatic phenotypes, while 22% of clutchmates from heterozygous intercrosses exhibited either a spherical (55%) or an intermediately shortened (45%) pancreas. n below bars represents the number of embryos examined from WT clutches (right), heterozygote in-crossed clutches (center), and embryos exhibiting donut-like pancreatic phenotypes (right). (G–I) donut mutants have a lesion in met. donut was mapped to a critical interval on Chr. 25 containing 14 annotated genes (G); met showed a T2324G variant (H) causing an L775R amino acid substitution (I). (J,K) Model structures of the human MET IPT3 domain with isoleucine 777 (analogous to zebrafish residue L775) marked green (J) or substituted arginine marked red (K). (L) Diagram of Met showing the I777R substitution localized to the high affinity HGF binding site in IPT3, and the furin cleavage site in the semaphorin-like domain. (M–P) Morpholino-mediated knockdown of met (M,N) or hgfa/b (O,P) resulted in phenocopy of the donut mutation. (Q) Dose dependence of morpholino-induced phenotypes. At 8 ng, metMO exhibited some non-specific developmental delay, suggesting toxicity. pen, penetrance; ex, expressivity; li, liver; pm, plasma membrane. EXPRESSION / LABELING:
PHENOTYPE:
|
|
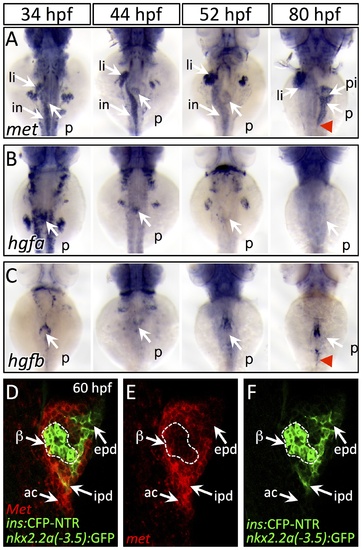
Dynamic expression of met and hgf during pancreas morphogenesis. (A) met is expressed strongly in the pancreatic bud (p), liver bud (li), and intestinal bulb (in) from 34–52 hpf. At 80 hpf, met expression is found throughout the exocrine pancreas and is diminished in the principal islet (pi). (B,C) hgfa (B) and hgfb (C) are expressed at 34–44 hpf near the dorsal pancreatic bud prior to pancreatic tail formation. At 52 hpf, at the onset of pancreatic tail formation, both hgfa and hgfb are expressed near the distal tip of the pancreatic bud, with hgfb expression persisting until the completion of pancreatic tail outgrowth (red arrowhead). (D–F) One-micron confocal plane images of duct:GFP;ins:CFP-NTR embryos stained for met mRNA using fluorescent in situ hybridization shows met expression (red) in both acinar cells (ac) and intrapancreatic ducts (ipd; green), but not in the principal islet (green outlined in white). Dorsal views, anterior to the top. beta-cells, β epd, extrapancreatic duct. EXPRESSION / LABELING:
|
|
Orthologous murine donut mutation, I776R, impairs HGF signaling and receptor trafficking. (A) Immunoblots of Met protein prepared from lysates of TOV112D cells transfected with murine MetWT or MetI776R. Upper panels blotted with anti-pY show diminished phosphorylation at tyrosines critical for signaling and lower panels blotted with anti-Met show loading controls. Precursor (white arrows) and mature (black arrows) forms of MetWT are both detected, but MetI776R is only present in the precursor form. (B) Quantification by densitometry of phosphorylated Met versus total Met in panel A (t test, * p<0,05; **p<0,01). (C–H) Immunofluorescence staining of HEK293 cells transfected with MetWT (C,F), MetI776R (D,G), or furin cleavage-incompetent MetR306A (E,H). Unfixed, unpermeabilized cells were stained with anti-Met ab1, which binds an extracellular epitope (C–E), and fixed, permeabilized cells were stained with ab2, which binds an intracellular epitope (F–H). MetWT and MetR306A are localized to the plasma membrane (C, E, insets in F, H), but MetI776R is not (D), and is mostly retained in the cytoplasm (G, inset). Intracellular staining of Met demonstrates similar transfection efficiency. DAPI staining (blue) marks nuclei. (I–J) Live imaging of zebrafish blastulae injected with zebrafish MetWT-mCherry (I) and MetL775R-mCherry (J) at 5 hpf. Zebrafish MetL775R-mCherry largely fails to localize to the plasma membrane as compared to MetWT. Scale bars, 20 μm. |
|
Cell migration, but not proliferation, is dysregulated in donuts908 mutants. (A–D) EdU incorporation assay in WT (A,C) and donuts908 mutant (B,D) animals at 56 (A,B) and 75 (C,D) hpf. (E) Quantification of proliferation assay data shows no significant change in acinar cell proliferation at early or late stages of pancreatic tail formation. (F–I) Morphology of exocrine tissues at 84 hpf in WT (F,H) and metMO-injected (G,I) larvae revealed by Tg(ptf1a:GFP) (acinar; F,G) or Tg(nkx2.2a(-3.5 kb):GFP) (duct; H,I) expression. In metMO-injected embryos, acinar and ductal cells fail to migrate caudally, and remain near the principal islet (β). In metMO larvae, ductal cells exhibit a more rounded morphology in the exocrine pancreas (insets) and are also observed in the liver region (asterisk). (J,K) 84 hpf WT (J) and metMO-injected (K) Tg(duct:GFP) larvae stained with Prox1 (blue) and Alcam (red). Pancreatic ductal cells are ectopically localized in the liver (li) along tracts of biliary ducts. (L) Quantification of pancreatic tail defects in small molecule treated larvae; inhibitors of the MAPK pathway (UO126, SB203580) and furin function (CMK) had little effect on pancreatic tail outgrowth, while inhibitors of PI3K (LY294002) and STAT3 (SU6656) function mimicked the type 1 and type 2 donut phenotypes. epd, extrapancreatic duct. EXPRESSION / LABELING:
PHENOTYPE:
|
|
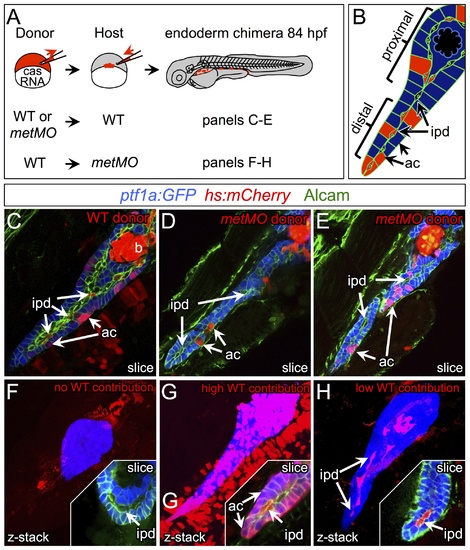
HGF/Met signaling is required in intrapancreatic ducts for pancreatic tail morphogenesis. (A) Schematic of cell transplantation experiments. WT or metMO-injected donor cells were transplanted into WT hosts (top), and WT donor cells were transplanted to metMO-injected hosts (bottom); cas mRNA injection biased donor cells toward endodermal differentiation. (B) Scheme used to identify and quantify contribution of transplanted cells in chimeras. Donor cells are marked by Tg(hs:mCherry) (red), acinar cells are marked by Tg(ptf:GFP)jh1 (blue), and Alcam immunostaining (green) delineates the ducts. (C–E) Single plane confocal images of WT (C) and metMO (D,E) donor endoderm transplanted into WT hosts: WT donor cells contributed to both intrapancreatic ductal (ipd) and acinar (ac) compartments, but metMO donor cells were excluded from the ducts. (F–H) WTkmetMO hosts: With no WT contribution to the pancreas, metMO morphants show donut-like phenotypes (E); with high contribution of WT donor cells, the pancreatic tail growth is rescued (G), and the tip of the tail is almost entirely comprised of WT donor cells (inset of single confocal slice); with moderate contribution of WT donor cells, the pancreatic tail morphology can be rescued with only WT ductal cells (H), and no WT acinar cells are observed in the tip (inset of single confocal slice). |
|
donut mutants variably lack muscle tone in pectoral fins. (A–C) 9 dpf Tg(ptf1a:GFP) larvae imaged from the dorsal aspect to show fin morphology (arrows). In WT larvae, fins are folded closely against the body (A), while in type 1 (B) and type 2 (C) donut mutant larvae, the pectoral fins lack tone, and display an open wing configuration. Bar = 0.5 mm. |
|
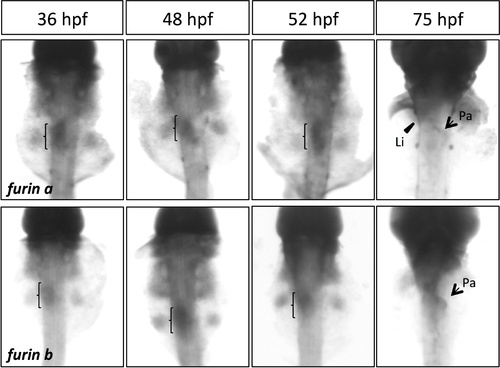
furina and furinb expression during pancreatogenesis by whole-mount in situ hybridization. Both furina and furinb are expressed in endoderm (brackets); furina is expressed in liver (Li) and pancreas (Pa) at 75 hpf. Dorsal views, anterior to the top. EXPRESSION / LABELING:
|
|
WT endoderm cannot rescue exocrine tail formation in hgfMO-injected larvae. (A–B) 84 hpf hgfMO-injected host larvae with a high contribution of transplanted WT endodermal cells (red). Extensive contribution of WT cells to both duct and acinar compartments did not rescue type 1 (A) or type 2 (B) exocrine pancreas tail outgrowth. |