- Title
-
Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development
- Authors
- Sacilotto, N., Monteiro, R., Fritzsche, M., Becker, P.W., Sanchez-Del-Campo, L., Liu, K., Pinheiro, P., Ratnayaka, I., Davies, B., Goding, C.R., Patient, R., Bou-Gharios, G., and De Val, S.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
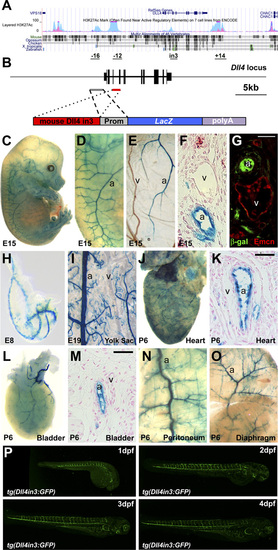
The Dll4in3 enhancer directs arterial-specific expression in both transgenic mice and zebrafish. (A) Schematic representation of mouse Dll4 gene (Upper) and Dll4in3hsp transgene (Lower). (B–L) The Dll4in3hsp transgene directs arterial-specific expression. Representative transgenic whole-mount embryos (B and D) and transverse sections (C and E) show reporter gene expression (X-Gal staining, blue) in dorsal aorta (da) but not the cardinal vein (cv). (Scale bar: 50 μm.) At E15, X-Gal staining is detected in arterial (a) but not venous (v) cells in whole-mount embryo (F and G), yolk sac (H), and transverse section (I). E15 transverse paraffin sections showed overlapping expression of X-gal staining (J), or β-gal (K and L) with the arterial markers Neuropilin1 (Nrp1) (J2) and Ephrin B2 (Efnb2) (K22). Expression of the venous marker Endomucin (Emcn) did not overlap that of the reporter gene (L22). (Scale bar: 10 μm.) (M–O) The mouse Dll4in3 transgene (M) directs arterial-specific expression of the GFP reporter gene in transgenic zebrafish line tg(Dll4in3:GFP) to dorsal aorta (da) and intersegmental arteries (ISA) but not the posterior cardinal vein (pcv) or intersegmental veins (ISVe)(N and O). (P–U) The zebrafish Dll4in3 transgene (P) directs arterial-specific expression in transgenic mice in arteries but not veins in whole-mount embryos (Q and S) and transverse sections (R and T). No overlap was seen with the venous marker Emcn (U). See also Fig. S1. EXPRESSION / LABELING:
|
|
Combinatorial knockdown of sox7, sox18, and rbpj in zebrafish results in ablation of both Dll4in3:GFP and endogenous dll4 expression. (A) Analysis of GFP expression in scrambled, rbpj, double sox7;sox18, and triple sox7;sox18;rbpj MO-injected tg(Dll4in3:GFP) embryos at 34 hpf. (B) Analysis of endogenous dll4 gene expression in scrambled, rbpj, double sox7;sox18, and triple sox7;sox18;rbpj MO-injected WT zebrafish embryos at 26 hpf. dll4 expression was detected by in situ hybridization. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos analyzed. In all cases, 0.125 pmol of sox7 and sox18 and 0.15 pmol of rbpj MO were used. *, highlights ectopic neural expression; arrowhead shows dorsal aorta. See also Fig. S6. |
|
Triple sox7;sox18;rbpj knockdown results in the loss of a detectable dorsal aorta. (A) Analysis of GFP expression in tg(fli1a:GFP) zebrafish embryos after injection of control (Upper) and triple sox7;sox18;rbpj MO (Lower) during the migration of angioblasts from the lateral plate mesoderm toward the midline and coalescence into a vascular cord. At 26 hpf, the dorsal aorta (red bracket) and posterior cardinal vein (blue bracket) can be detected in the control MO-injected embryo but not in the triple MO (white bracket highlights single axial vessel). See also Fig. S7 A and B. (B) Lateral view (Upper) and optical transversal sections (Lower, location indicated by white bar) of control, rbpj, sox7;sox18, and sox7;sox18;rbpj MO-injected fli1a:EGFP embryos at 2 dpf. See also Movies S1, S2, S3, and S4 for 3D animations of each embryo. (C–E) Whole-mount in situ hybridization of venous (C) and arterial (D and E) markers on control, rbpj, double sox7;sox1,8 and triple sox7;sox18;rbpj MO at 26 hpf. See also Fig. S8 A–D. Black arrowhead, dorsal aorta; white arrowhead, where dorsal aorta should be; black arrow, posterior cardinal vein; white bracket, single axial vessel; black bracket, notochord. Probes used are depicted in the bottom left of each picture. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos analyzed. In all cases, 0.125 pmol of sox7 and sox18 and 0.15 pmol of rbpj MO were used. EXPRESSION / LABELING:
PHENOTYPE:
|
|
(A) Schematic representation of the Dll4 locus from University of California, Santa Cruz (UCSC) ENCODE Browser (1), HUVEC-specific H3K27Ac in light blue, K562 leukemia cell line in pink. On the bottom, the potential vascular-specific enhancers are depicted with their respective positions (in kb) with respect to the TSS (except for the intron 3, indicated as in3). (B) Schematic representation of the mouse Dll4 locus (Upper line, exons are black boxes) and Dll4in3end transgene (Lower line). Prom, endogenous promoter. (C–G) A representative X-Gal–stained E15 Dll4in3end transgenic embryo. X-Gal staining is detected in arterial (a) but not venous (v) cells in whole-mount embryo (C and D), yolk sac (E), and transverse section (F). Expression of the venous marker Endomucin (Emcn) did not overlap that of the reporter gene (G). (H–O) Further examples of transgenic mice expressing the mouse Dll4in3hsp transgene at E8 (H), E19 (I), and tissues from a P6 pup (J–O), all showing X-Gal staining in arteries (a) but not veins (v). (P) Time course of the tg(Dll4in3:GFP) zebrafish line between 1–4 dpf. |
|
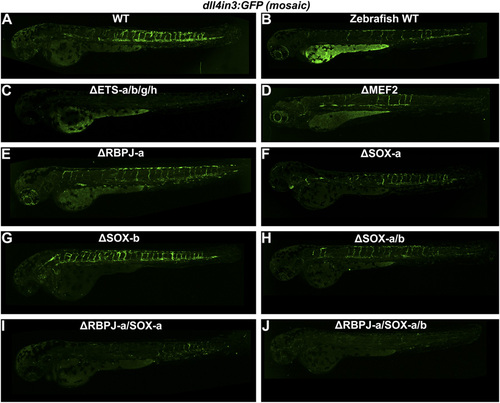
(A–J) Representative 2–2.5 dpf Tol2-mediated mosaic transient transgenic zebrafish generated using WT or mutant versions of the Dll4in3:GFP transgene. (A) mouse WT Dll4in3:GFP, (B) zebrafish WT dll4in3:GFP, (C) ΔETS-a/b/g/h Dll4in3:GFP, (D) ΔMEF2C Dll4in3:GFP, (E) ΔRBPJ-a Dll4in3:GFP, (F) ΔSOXa Dll4in3:GFP, (G) ΔSOX-b Dll4in3:GFP, (H) ΔSOX-a/b Dll4in3:GFP, (I) ΔRBPJ-a/SOX-a Dll4in3:GFP, (J) ΔRBPJ-a/SOX-a/b Dll4in3:GFP. |
|
(A) Analysis of GFP expression in 3-dpf tg(Dll4in3:GFP) zebrafish embryos after scrambled, rbpj, double sox7;sox18, and triple sox7;sox18;rbpj MO injections. Triple sox7;sox18;rbpj MO results in total ablation of tg(Dll4in3:GFP) expression. We used 0.25 pmol for sox7 and sox18 and 0.30 pmol of rbpj MO. (B–E) Analysis of GFP and endogenous dll4 expression in tg(Dll4in3:GFP) (34 hpf) and WT zebrafish (26 hpf) after chemical inhibition of Notch signaling with 100 μM N-[N-3,5-Difluorophenacetyl]-L-alanyl-S-phenylglycine Methyl Ester (DAPM) alone (B and C) and in combination with sox7;sox18 MO (D and E). sox7; sox18 MO injection in combination with DAPM results in total ablation of both GFP and endogenous dll4 expression. (F–I) Chemical inhibition of VEGF signaling in tg(Dll4in3:GFP) zebrafish embryos with 1 μM SU5416 alone or in combination with rbpj or double sox7;sox18 MO abolishes the GFP expression. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype/ total number of embryos analyzed. In all cases, 0.125 pmol of sox7 and sox18 and 0.15 pmol of rbpj MO were used. |
|
(A) Whole-mount in situ hybridization against fli1a in 26 hpf WT zebrafish embryos after injection of control (Left) and triple sox7;sox81;rbpj MO (Right). Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos. (B) Analysis of GFP expression in 26 hpf tg(fli1a:GFP) zebrafish embryos after rbpj and sox7;sox18 MO injection. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos. (C and D) Whole-mount double immunofluorescence against activated caspase- 3 (red) and GFP on tg(fli1a:GFP) zebrafish embryos after control (Left) and triple sox7;sox18;rbpj MO (Right) injection at 21 hpf (C) and 26 hpf (D). Nuclei were counterstained with DAPI. No significant apoptosis was detected. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos. (E) Analysis of GFP expression in 2.5-dpf tg(kdrl:GFP);tg(gata1:dsRed) zebrafish embryos after control and sox7;sox18;rbpj MO injection. Pooled blood cells could be detected in the trunk and tail of the sox7;sox18;rbpj MO-injected fish. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos. In all cases, 0.125 pmol of sox7 and sox18 and 0.15 pmol of rbpj MO were used. PHENOTYPE:
|
|
(A–D) Whole-mount in situ hybridization of arterial markers on control, rbpj, double sox7;sox18, and triple sox7;sox18;rbpj MO at 26 hpf. Triple sox7; sox18;rbpj knockdown results in the loss of all of the arterial markers analyzed. Black arrowhead shows dorsal aorta. White arrowhead indicates where dorsal aorta should be. Black arrow indicates posterior cardinal vein, and white bracket indicates single axial vessel/expanded posterior cardinal vein. Black bracket indicates notochord. Probes used are depicted in the bottom left of each picture. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype/total number of embryos analyzed. In all cases, 0.125 pmol of sox7 and sox18 and 0.15 pmol of rbpj MO were used. (E–K) Whole-mount in situ hybridization of arterial (A–F) and venous (G) markers on control and dll4 MO-injected WT zebrafish embryos at 26 hpf. Probes are depicted in the bottom left. Values on the bottom right indicate number of embryos with the predominant and displayed phenotype per total number of embryos. Six nanograms of dll4 MO were used. |