- Title
-
Dner inhibits neural progenitor proliferation and induces neuronal and glial differentiation in zebrafish
- Authors
- Hsieh, F.Y., Ma, T.L., Shih, H.Y., Lin, S.J., Huang, C.W., Wang, H.Y., and Cheng, Y.C.
- Source
- Full text @ Dev. Biol.
|
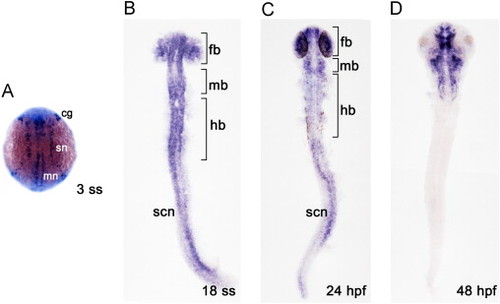
dner expression in the developing zebrafish. Expression of dner was detected during zebrafish embryogenesis by in situ hybridization. The stages of embryos are shown in the bottom left corner of each panel. All panels show dorsal views, with anterior to the top. (A) dner expression in proneuronal clusters, as 3 rostrocaudal strips. (B–C) Differing levels of dner expression were observed in differentiating neurons in the brain and spinal cord. (D) Expression was retained in the brain; however, it was lost in the spinal cord at 48 hpf. cg, cranial ganglia; fb, forebrain; hb, hindbrain; mb, midbrain; mn, motor neurons; sn, sensory neurons; scn, spinal cord neurons. EXPRESSION / LABELING:
|
|
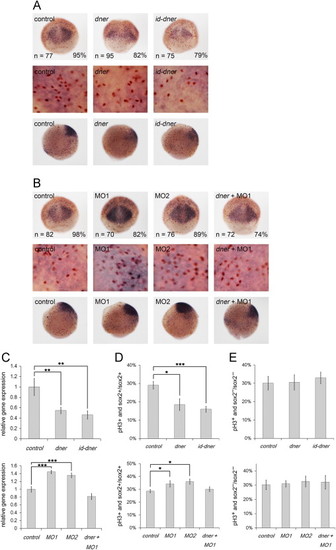
Dner inhibits the proliferation of neural progenitors. Proliferation of neural progenitors was detected by in situ hybridization using a sox2 riboprobe (purple) and counterstaining with phosphohistone H3 antibody (brown) at 75% epiboly. (A–B) Top and middle panels are dorsal and bottom panels are lateral views. Middle panels show enlarged images of the corresponding top panels. (A) The proliferation of neural progenitors was reduced in dner- or id-dner-injected embryos compared with the controls. (B) Injection with either MO1 or MO2 induced the proliferation of neural progenitors, and the phenotypes caused by morpholino injection could be rescued by concomitant injection with dner cRNA. (C) The results of in situ hybridization were confirmed quantitatively by qPCR analysis. (D) The proportions of phosphohistone H3- and sox2-positive cells among the total sox2-positive cells were quantified. (E) The proportions of phosphohistone H3-positive and sox2-negative cells in sox2-negative cells counted in adjacent surface ectoderm showed no significant deviation in Dner-distorted embryos. N, p<0.05; NN, p<0.01; NNN, p<0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
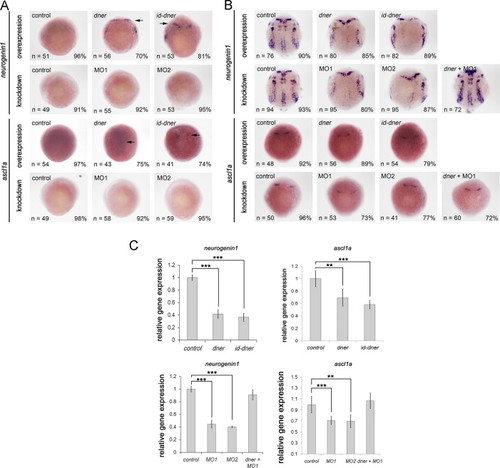
Dner induces premature differentiation of neuronal precursors. Embryos were examined by in situ hybridization using neurogenin1 and ascl1a. (A) At 75% epipoly, ectopic neurogenin1 and ascl1a were detected in dner or id-dner-injected embryos (arrows). (B) At the bud stage, the expression levels of neurogenin1 and ascl1a were significantly decreased in dner or id-dner-injected embryos in comparison with the controls. Injections with either MO1 or MO2 also reduced the expression of these proneural markers at the bud stage. The phenotypes caused by morpholino injection could be rescued by concomitant injection with dner cRNA. (C) qPCR analysis confirmed the results obtained by in situ hybridization. NN, p<0.01; NNN, p<0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
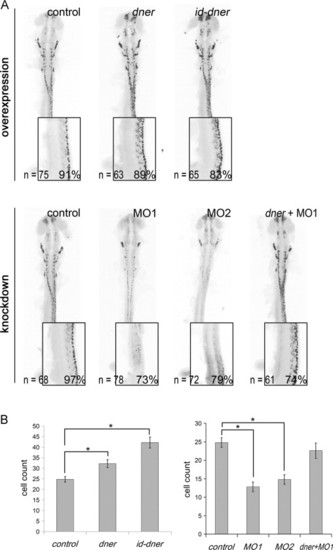
Dner-induced neuronal differentiation and dner morpholinos are sufficient to inhibit differentiation. Embryos were analyzed by immunohistochemistry using anti-Hu antibody at 24 hpf. The black-and-white fluorescent signals were inverted using negative film for a better presentation. (A) Injections with either dner or id-dner increased the frequency of HuC/D-positive cells. Injections with either morpholino were sufficient to decrease the HuC/D signals. This reduction could be rescued by co-injection with dner cRNA. The insets in each panels show lateral view enlargements of the 3-somite to 9-somite levels of the spinal cord. HuC/D-positive cells were counted in this region and quantified in (B). N, p<0.05. |
|
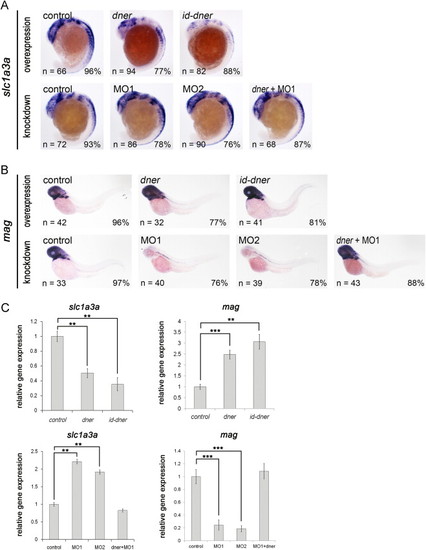
Dner inhibits the expression of glial precursors and induces the formation of mature glial cells. (A) Expression of slc1a3a was reduced in embryos that overexpressed dner or id-dner at the 18-somite stage. Injections with either MO1 or MO2 upregulated the expression of slc1a3a, and this effect could be rescued by concomitant injection with dner cRNA. (B) Overexpression of dner or id-dner induced the expression of mag at 3 dpf. In contrast, mag expression was significantly downregulated in the morpholino-injected embryos, and this effect was rescued by co-injection with dner cRNA. (C) qPCR analysis confirmed the results of the in situ hybridization shown in A–B. NN, p<0.01; NNN, p<0.001. EXPRESSION / LABELING:
|
|
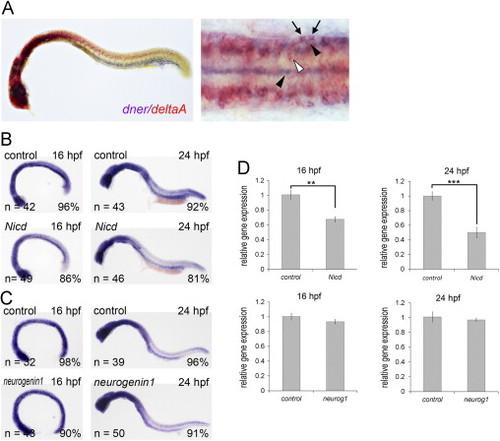
Expression of dner partially overlaps with that of deltaA and can be inhibited by Notch activation. (A) Double in situ hybridization showing that the expression of dner (purple, black arrowheads) and deltaA (red, white arrowhead) partially overlap (arrows). (B) Expression of dner was downregulated by Notch intracellular domain (Nicd). (C) Overexpression of neurogenin1 did not lead to significant alteration in dner expression. (D) These results in B and C were confirmed by qPCR analysis. NN, p<0.01; NNN, p<0.001. EXPRESSION / LABELING:
|
|
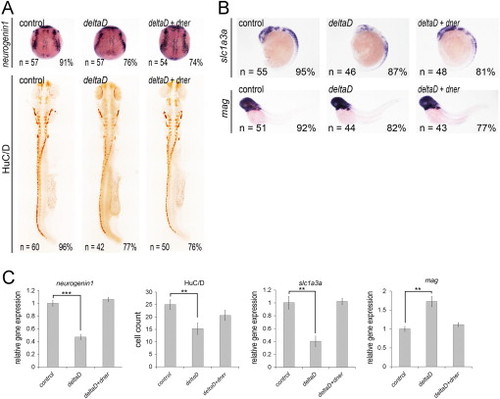
Co-injection of dner nullifies the effects of deltaD overexpression. (A) Overexpression of deltaD caused decreased neurogenin1 and HuC/D expression. In contrast, injection of deltaD downregulated slc1a3a and upregulated mag expression (B). These phenotypes were restored by co-injection of dner. (C) qPCR analysis confirmed the results of in situ hybridization. NN, p<0.01; NNN, p<0.001. |
|
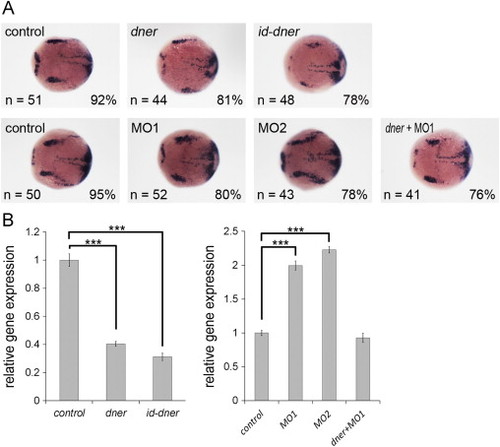
Dner inhibits her4 expression. (A) her4 expression was downregulated in dner or id-dner-injected embryos and was increased in dner morphants. (B) This result was confirmed by qPCR. NN, p<0.001. EXPRESSION / LABELING:
|
|
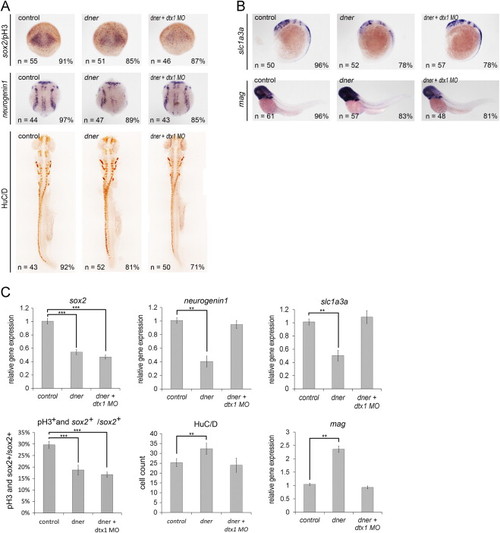
Dner is mediated by Deltex1 in neuronal and glial cells but not in neural progenitors. (A) dner injection decreased the proliferation of neural progenitors, and this could not be restored by co-injection with deltex1 morpholino. (A–B) dner overexpression induced neuronal and glial differentiation, and these effects were abolished by deltex1 knockdown. (C) qPCR analysis confirmed the results of the in situ hybridization shown in A–E. NN, p<0.01; NNN, p<0.001. |
|
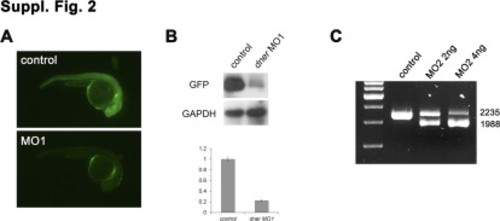
(A) Embryos co-injected with 5? dner-EGFP cRNA and control morpholino showed strong EGFP expression (upper panel). In contrast, the EGFP signal was abolished in embryos co-injected with 5? dner-EGFP cRNA and dner MO1 (lower panel). (B) The levels of GFP expression were confirmed by Western blot analysis and quantified. (C) The efficacy of MO2 was validated by RT-PCR. Wild-type dner mRNA produced a 2235-bp PCR product, while alternatively spliced transcripts from morphant embryos yielded a 1988-bp fragment. |
|
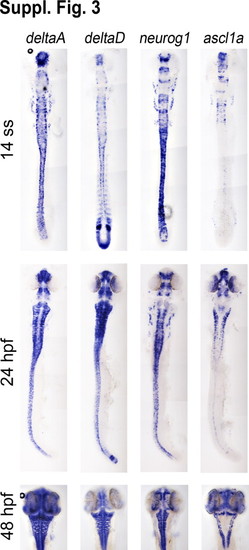
Expression comparison of deltaA, deltaD, neurogenin1, and zash1 in the developing nervous system. |
Reprinted from Developmental Biology, 375(1), Hsieh, F.Y., Ma, T.L., Shih, H.Y., Lin, S.J., Huang, C.W., Wang, H.Y., and Cheng, Y.C., Dner inhibits neural progenitor proliferation and induces neuronal and glial differentiation in zebrafish, 1-12, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.