- Title
-
Fgf signaling governs cell fate in the zebrafish pineal complex
- Authors
- Clanton, J.A., Hope, K.D., and Gamse, J.T.
- Source
- Full text @ Development
|
Fgf components are expressed in the epithalamus during parapineal formation in zebrafish. (A-C) fgf8a (red) is expressed in the anterior pineal complex (white arrowheads) of foxd3:GFP/flhBAC:Kaede (green)-expressing embryos during parapineal development. A, anterior; L, left; P, posterior; R, right. (A′-C′) Optical cross sections at the level of the dashed lines in A-C, respectively. fgf8a is expressed in the ventral region of the anterior pineal complex anlage (white arrowheads). (D-F) Dorsal views of in situ hybridizations of fgf17 (blue) relative to the pineal complex (otx5, red). fgf17 is expressed in the anterior pineal complex (black arrowheads) at 24 hpf, but is almost gone by 30 and 36 hpf. Dashed line indicates plane of sectioning. (D′-F′) Cryosections at the levels of the dashed lines in D-F, respectively. fgf17 expression is evident in the region ventral to the anterior pineal complex anlage (black arrows) at 24 hpf. (G-I). Confocal projections show that fgfr4 (red) is expressed within the pineal complex anlage including the anterior portion (white arrowheads). (G′-I′) Optical cross sections at the level of the dashed lines in G-I, respectively, showing fgfr4 expresseion in the anterior pineal complex anlage where parapineal precursors are located (white arrowheads). (J-L) Dorsal views of in situ hybridization of erm (blue) relative to the pineal complex anlage (otx5; red). erm expression shows that Fgf signaling activity is high in the anterior pineal complex (white arrowheads). White dashed lines indicate the plane of sectioning. (J′-L′) Cryosections at the level of the dashed lines in J-L, respectively, of in situ hybridizations of erm. erm is detected in the anterior pineal complex anlage at all stages (black arrowheads). Scale bar: 25 μm. |
|
Loss of Fgf signaling results in fewer parapineal cells. (A,B) Dorsal views of WT and fgf8ax15 mutant zebrafish embryos labeled with gfi-1.2 (parapineal cells; black arrowheads). (C,D) Dorsal views showing expression of gfi-1.2 (black arrowheads) in WT larvae at 52 hpf that were treated between 24 and 30 hpf with either DMSO or SU5402. In A-D, dashed line encircles the pineal complex anlage. (E) fgf8ax15 mutants displayed a significant reduction in parapineal cell number compared with WT (***P=<0.0005 by t-test). (F) Reductions in parapineal cell numbers were observed in embryos treated with SU5402, particularly for 18-24 hpf and 24-30 hpf treatments (***P<0.0005 by t-test). Error bars represent s.e.m. Scale bar: 25 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Fgf8a overexpression is not sufficient to induce supernumerary parapineal cells. (A,B) Dorsal view of non-transgenic and Tg[hps70:fgf8a]b193 zebrafish larvae at 52 hpf that were induced for 30 minutes at 24 hpf and labeled with gfi-1.2 (black arrowheads). Dashed line encircles the pineal complex anlage. (C) Graph quantifying the number of gfi-1.2-expressing cells. Error bars represent s.e.m. n.s., not significant. Scale bar: 25 μm. EXPRESSION / LABELING:
|
|
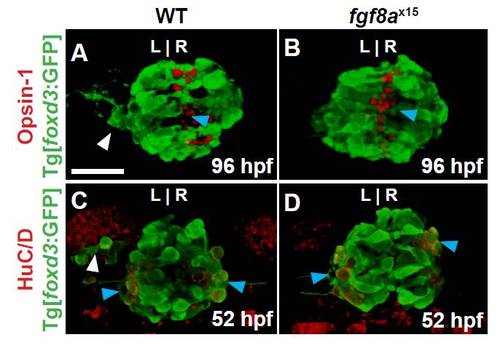
fgf8ax15 mutants have a selective increase in cone photoreceptor number compared with WT larvae. (A) Schematic of the pineal complex of the larval zebrafish at 52 hpf. (B-E) Dorsal (B,C) and frontal (D,E) views of WT and fgf8ax15 mutants labeled with foxd3:GFP (pineal complex) and Arr3a (cone photoreceptors) at 52 hpf. fgf8ax15 mutants lack a distinct parapineal organ compared with WT (white arrowheads). L, left; R, right. (F,G) fgf8ax15 and WT embryos have equivalent numbers of foxd3:GFP expressing cells (F), projection neurons (HuC/D) and rod photoreceptors (Opsin1) (G). However, fgf8ax15 mutants have more cone photoreceptors (Arr3a) in the pineal complex than WT (G) (**P<0.005 by t-test). Error bars represent s.e.m. Scale bar: 25 μm. |
|
Cell fate analysis of the anterior pineal complex indicates that parapineal precursors adopt a cone photoreceptor fate in fgf8ax15 mutants. (A) Fate analysis scheme. Green, fluorescein-positive cells; red, Arr3a-positive cells; yellow, cells expressing both fluorescein and Arr3a. (B-C′) Dorsal views of serial confocal sections in the pineal complex of WT (B,B2) or fgf8ax15 (C,C′) zebrafish embryos at 52 hpf, after labeling of the anterior pineal complex anlage at 24 hpf. In WT, clusters of labeled parapineal cells are evident (blue arrowheads). In fgf8a mutants, many of the cells that were labeled in the anterior pineal complex anlage at 24 hpf become cone photoreceptors (blue arrowheads). (D) When Fgf signaling is reduced, a significantly higher percentage of labeled cells become cone photoreceptors compared with controls (**P<0.005 by t-test). Error bars represent s.e.m. Scale bar: 30 μm. PHENOTYPE:
|
|
Ectopic cone photoreceptors persist in flh-depleted fgf8ax15 mutants. (A,B) Dorsal views of 96 hpf WT and fgf8ax15 mutant zebrafish embryos injected with flh morpholino (flhMO) labeled with foxd3:GFP (pineal complex) and Arr3a (cone photoreceptors). In WT injected with flhMO, a full-sized parapineal organ (dotted line) is evident, but pineal cells are reduced in number. In flhMO-injected fgf8ax15 mutants, no parapineal organ is visible. (C) The number of foxd3:GFP-expressing cells is unchanged between fgf8ax15;flhMO and WT;flhMO. However, the number of cone photoreceptors increased in fgf8ax15;flhMO relative to WT;flhMO larvae (**P<0.005 by t-test). Error bars represent s.e.m. n.s., not significant. Scale bar: 25 μm. EXPRESSION / LABELING:
|
|
Fgf8a and Tbx2b act additively in the formation of parapineal cells, whereas Fgf8a acts downstream of Tbx2b to prevent the formation of cone photoreceptors. (A-D) Dorsal views of uninjected (NI) WT (A), uninjected fgf8ax15 mutant zebrafish larvae (B), WT injected with tbx2bMO (C) and fgf8ax15 mutant embryos injected with tbx2bMO (D) at 96 hpf, labeled for gfi-1.2 to mark parapineal cells (arrowheads). (E) Many fewer parapineal cells are detected when tbx2b and fgf8a are simultaneously reduced than when either is singly depleted (**P<0.005, ***P<0.0005). (F-I) Dorsal views of confocal slices of WT;NI (F), fgf8ax15;NI (G), WT;tbx2bMO (H) and fgf8ax15 ;tbx2bMO (I) larvae labeled with foxd3:GFP to mark the entire pineal complex and Arr3a to mark cone photoreceptors. (J) Tbx2b depletion reduces the number of cone photoreceptors to a level indistinguishable from non-injected WT and tbx2b morphants (***P<0.0005). Error bars represent s.e.m. Scale bars: 25 μm. |
|
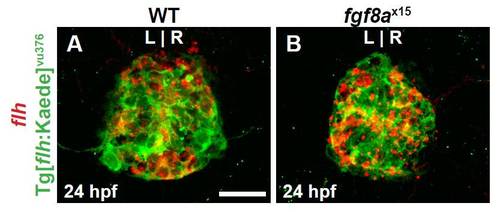
Tg[flhBAC:kaede]vu376 recapitulates endogenous flh expression. (A,B) Dorsal view of confocal slices of WT and fgf8ax15 mutants showing flh mRNA and Tg[flhBAC:kaede]vu376 in the pineal complex anlage at 24 hpf. Tg[flhBAC:kaede]vu376 and flh expression largely overlap in both WT and mutants confirming that the transgene is faithful to flh and that fgf8ax15 mutants. Scale bar: 25 μm. |
|
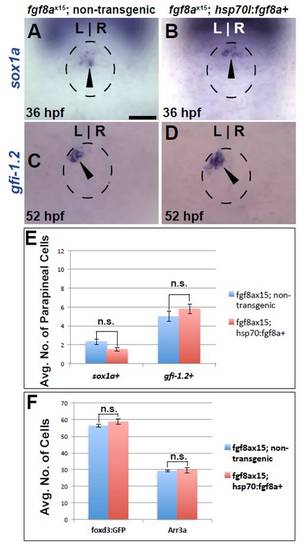
sox1a is expressed in parapineal cells near their time of differentiation. All images are from dorsal view. (A) sox1a expression (black arrowhead) in WT pineal complex anlage (dashed circle). (B) A cluster of sox1a-expressing cells (black arrowhead) are in the anterior pineal complex anlage of 28 hpf WT embryos. (C) By 36 hpf, sox1a expression is present (black arrowhead) to the left of the pineal anlage suggesting that it is indeed expressed in parapineal cells. (D) Confocal slice of 48 hpf WT larvae showing colocalization of sox1a (white arrowhead) and Tg[foxd3:GFP] confirming that sox1a is expressed in parapineal cells. (E,F) sox1a expression (black arrowheads) in WT and fgf8ax15 mutants at 32 hpf. (G) Graph quantifying the number of sox1a-expressing cells in WT and fgf8ax15 mutants at 32 hpf. fgf8ax15 mutants have significantly reduced numbers of sox1a-expressing cells (***P<0.0005 by t-test). Error bars represent s.e.m. Scale bar: in A, 20 μm; in B, 25 μm; in C: 20 μm. |
|
Fgf signaling is needed in a sustained manner during parapineal differentiation. (A,B) Dorsal views of gfi-1.2 labeling (black arrowheads) in 52 hpf embryos treated with DMSO and SU5402 from 24 to 30 hpf. Dashed line encircles pineal complex anlage. (C) Graph quantifying the number of gfi-1.2-expressing cells (*P<0.05, ***P<0.0005). Error bars represent s.e.m. Scale bars: 20 μm. |
|
Parapineal differentiation is not dependent on migration. (A,B) 3D reconstructions of the pineal complex of DMSO- and 6 μM SU5402-treated larvae at 52 hpf. The parapineal (white arrowheads) fails to migrate away from the midline (dashed line) in SU5402-treated larvae. (C) Graph quantifying the number of sox1a-expressing cells. The number of sox1a-expressing cells is unchanged between DMSO- and SU5402-treated embryos. (D) Graph quantifying the distance of parapineal cell migration. Parapineal cell migration is greatly attenuated in SU5402-treated larvae (***P<0.0005 by t-test). Error bars represent s.e.m. n.s., not significant. Scale bar: 25 μm. |
|
Fgf8a overexpression cannot rescue parapineal cell number defects in fgf8ax15 mutants. (A,B) Dorsal views of sox1a expression (black arrowheads) in fgf8ax15 mutants with and without the Tg[hsp70l:fgf8a]b1198 transgene at 36 hpf. (C,D) Dorsal views of gfi-1.2 expression (black arrowheads) in fgf8ax15 mutants with and without the Tg[hsp70l:fgf8a]b1198 transgene 52 hpf. Dashed line encircles pineal complex anlage in A-D. (E) Graph quantifying number of sox1a- and gfi-1.2-expressing cells in fgf8ax15 mutants with and without the Tg[hsp70l:fgf8a]b1198 transgene. (F) Graph quantifying the number of Tg[foxd3:GFP]- and Arr3a-positive cells in fgf8ax15 mutants with and without the Tg[hsp70l:fgf8a]b1198 transgene. Error bars represent s.e.m. n.s., not significant. Scale bar: 20 μm. |
|
fgf8a mutants exhibit no change in the number of rod photoreceptors or projection neurons. All images are dorsal views of confocal 3D reconstructions. WT larvae were co-labeled with foxd3:GFP and either Opsin-1 (rod photoreceptors outer segment) at 96 hpf or HuC/D (projection neurons) at 52 hpf. (A) Wild-type larvae exhibiting a parapineal (white arrowhead) and rod photoreceptors (blue arrowhead). (B) fgf8ax15 mutants have no parapineal organ but still form rod photoreceptors (blue arrowhead). (C) Wild-type embryos with visible parapineal (white arrowhead) and projection neurons (blue arrowheads). (D) fgf8ax15 mutants still develop projection neurons (blue arrowheads). Scale bar: 25 μm. |
|
Selective increase in cone cells persists in fgf8ax15 mutant and SU5402-treated larvae. (A,B) Dorsal views of WT and fgf8ax15 mutants labeled with foxd3:GFP (pineal complex) and Arr3a (cone photoreceptors) at 96 hpf. fgf8ax15 mutants lack a distinct parapineal organ compared with WT (white arrowhead). (C) Graph quantifying the number of foxd3:GFP- and Arr3a-positive cells in wild-type and fgf8ax15 embryos at 96 hpf. fgf8ax15 and wild-type embryos have equivalent numbers of foxd3:GFP-expressing cells. However, fgf8ax15 mutants have more cone photoreceptors in the pineal complex than are seen in WT. (D,E) Dorsal views of DMSO- and SU5402-treated embryos with parapineal. SU5402-treated embryos lack obvious parapineal (white arrowhead). (F) Graph quantifying the number of Tg[foxd3:GFP] and Arr3a cells in 52 hpf larvae treated with DMSO or 12 μM SU5402 from 24 to 30 hpf. (***P<0.0005 by t-test). Error bars represent s.e.m. n.s., not significant. Scale bar: 25 μm. |
|
Tbx2b and Fgf8a have no cross-regulatory relationship. (A,B) Dorsal views of DMSO- or SU5402 treated embryos labeled with tbx2b at 30 hpf. Embryos were treated from 24 to 30 hpf. (C-E) Dorsal view of erm expression in the pineal complex of WT, tbx2bc144 mutant and fgf8ax15 mutant embryos at 30 hpf. The position of the pineal is indicated by the dashed circle. Scale bars: 25 μm. |