- Title
-
BMP signaling and spadetail regulate exit of muscle precursors from the zebrafish tailbud
- Authors
- O'Neill, K., and Thorpe, C.
- Source
- Full text @ Dev. Biol.
|
Spt and chd expression are required for tail somite formation. Live embryos were photographed at 24 hpf. All images are lateral views with left being anterior and posterior to the right. WT embryos have somites throughout the trunk and tail (A). spt mutants lack trunk somites and have an enlarged tailbud (B). chd embryos are ventralized and have reduced trunk somites(C). spt and chd were crossed to see if spt phenotype could be rescued. Instead chd;spt mutants exhibit an even larger tailbud and no visible somites (D). PHENOTYPE:
|
|
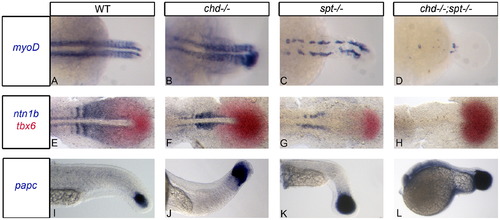
Chd;spt mutants produce mesodermal progenitor cells in the tailbud but are not able to form somites. (A)–(D) Dorsal view of myoD expression in 24 hpf embryos. (A)–(C) WT, chd, and spt embryos form organized somites even if it is only in the tail (spt). chd;spt mutants do not form organized somites. (60/65 have no organized somites. The remaining 5 only had 2–3 somites form.) (D). (E)–(H), dorsal view of ntn1b expressing muscle cells and tbx6 expressing MPCs in 11 hpf embryos. (E)–(G) WT, chd, and spt embryos have MPCs in tailbud as well as differentiated muscle cells outside the tailbud. chd;spt embryos have an accumulation of MPCs in the tailbud and no differentiated muscle cells. (I)–(L), lateral view of papc expression in 24 hpf embryos. WT and mutant embryos have MPCs in the tailbud with spt and chd;spt embryos having a large accumulation of progenitor cells. EXPRESSION / LABELING:
PHENOTYPE:
|
|
BMP inhibition is required for cells to exit the tailbud and form somites. P-smad 1/5/8 antibody was used to detect BMP activity in the tailbud of embryos during somitiogenesis. (A)–(D) dorsal view of P-smad 1/5/8 antibody fluorescence in 17 hpf (16 som) embryos; (E)–(G) are lateral views of P-smad 1/5/8 staining in 17 hpf embryos. WT embryos have normal levels of active BMP ((A) and (E)). Chd-/- (B) and oepMO (F) embryos have elevated levels of BMP, possibly due to the roles of chd and oep in BMP inhibition. chd-/-;spt-/- (D) and oepMO;sptMO (G) embryos also have higher levels of BMP in the tailbud, however, elevated levels are found in only some areas of the tailbud. Elevated Bmp activity and lack of spt leads to phenotypes where progenitor cells cannot exit the tailbud to form somites. (H)–(I), dorsal view of myoD expression in 24 hpf embryos: chd;spt embryos treated with dorsomorphin, a small molecule inhibitor of BMP, at 12 hpf were partially rescued and able to produce tail somites. (23/25 had 5–9 somites) (I), whereas untreated chd;spt embryos do not produce somites (1/26 had 5–9 somites) (H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Ability to exit the tailbud is a cell autonomous fate decision. Cell transplants were performed at 30–50% epiboly stages as diagramed above. Labeled donor cells were placed on the ventral lateral margin of unlabeled host embryos. (A)–(D), Live embryos were photographed using confocal microscopy at 30–48 hpf. (E)–(H), Recipient embryos were scored based on the most anterior somite containing donor cells. Somite 1 being the most anterior and 31 being the most posterior. Cells injected with caBMPR mRNA 4 μg/mL and placed in a WT host were able to exit the tailbud and differentiate in posterior and anterior tail somites, n=9 ((A) and (E)). Cells injected with sptMO 3 μg/mL were also able to leave the tailbud in WT background and contribute to tail somites, n=20 ((B) and (F)). Cells injected with caBMPR mRNA +sptMO were not able to efficiently leave the tailbud in WT backgrounds. A few donor cells could be occasionally found in posterior tail somites and 3 embryos had donor cells in anterior tail somites. However, the majority of transplanted cells were still in the tailbud at 48 hpf, n=14 ((C) and (G)). When chdMO cells were placed in a chdMO;sptMO background they were able to exit the tailbud and migrate anteriorly, n=15 ((D) and (H)). |
|
Ability to exit the tailbud is a cell autonomous fate decision. Cell transplants were performed at 30–50% epiboly stages as diagramed above. Labeled donor cells were placed on the ventral lateral margin of unlabeled host embryos. (A)–(D), Live embryos were photographed using confocal microscopy at 30–48 hpf. (E)–(H), Recipient embryos were scored based on the most anterior somite containing donor cells. Somite 1 being the most anterior and 31 being the most posterior. Cells injected with caBMPR mRNA 4 μg/mL and placed in a WT host were able to exit the tailbud and differentiate in posterior and anterior tail somites, n=9 ((A) and (E)). Cells injected with sptMO 3 μg/mL were also able to leave the tailbud in WT background and contribute to tail somites, n=20 ((B) and (F)). Cells injected with caBMPR mRNA +sptMO were not able to efficiently leave the tailbud in WT backgrounds. A few donor cells could be occasionally found in posterior tail somites and 3 embryos had donor cells in anterior tail somites. However, the majority of transplanted cells were still in the tailbud at 48 hpf, n=14 ((C) and (G)). When chdMO cells were placed in a chdMO;sptMO background they were able to exit the tailbud and migrate anteriorly, n=15 ((D) and (H)). PHENOTYPE:
|
|
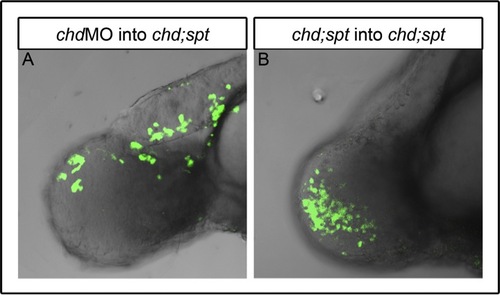
Controls for mGFP cell transplantation experiments further indicate tailbud exit is a cell autonomous function. Lateral view of 48 hpf embryos: chd MO cells in double mutant (chdMO;sptMO) background were able to mix with other cells in tailbud and some were able to exit the tailbud ((A), n=2). Double (chdMO;sptMO) mutant cells in double mutant background could intermix with other cells in the tailbud but, as expected, were not able to leave the tailbud ((B), n=2). |
|
Length and width measurements in tailbud cells. Top down view of tailbud cells in a chd-/- embryo 14 hpf: Cells just posterior of the notochord were used to calculate length to width ratios of WT and mutant embryos. Length was measured perpendicular to the notochord, while width was measured parallel to the notochord. Cell measurements were made using ImageJ draw and measure tools (Rasband, 1997-2009). |
Reprinted from Developmental Biology, 375(2), O'Neill, K., and Thorpe, C., BMP signaling and spadetail regulate exit of muscle precursors from the zebrafish tailbud, 117-127, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.