- Title
-
Suppression of rap1 impairs cardiac myofibrils and conduction system in zebrafish
- Authors
- Dong, W., Yang, Z., Yang, F., Wang, J., Zhuang, Y., Xu, C., Zhang, B., Tian, X.L., and Liu, D.
- Source
- Full text @ PLoS One
|
Rap1 knock-down led to abnormal heart and caudal fin development in zebrafish. Ubiquitous expressions of (A) rap1a and (B) rap1b were shown at 24 hpf. rap1MO injection led to the heart and caudal fin-fold phenotypes and curved trunk (arrows in C) at 48 hpf compared to wild type fish, and rap1 mRNA partially rescued the heart malformation in rap1 animals (C). The statistics indicates the proportion of fish embryos with heart phenotype was significantly decreased by rap1 mRNA rescue (D). mp157e fish was used to show the heart morphology of wild type, morphant and rescued morphant at 48 hpf at a higher magnification, the atrium (red), ventricle (green) and cardiocoelom (white) are marked by color dotted lines (E). In rap1MO larvae, the heart apparently failed to develop properly, while rap1 mRNAs partially restored the heart looping failure. Scale bar, 150 µm in A, B; 250 µm in C; 100 µm in E. Columns and error bars in D show mean±S.D. n>300 in each group. |
|
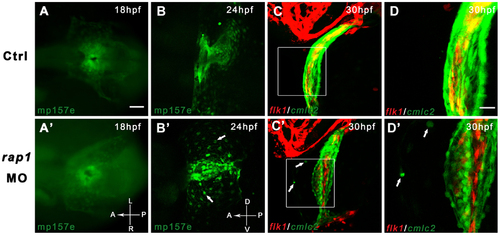
Time-lapsed observation revealed abnormal heart development in Rap1 knock-down zebrafish. Transgenic fish mp157e without any injection (A) and injected rap1MO (A′) were indistinguishable in early heart development. By 24 hpf, rap1MO injected mp157e embryos, crossed in flk1:mCherry background, started to show abnormality in heart development and remodeling (B to B′), including the failure of heart tube extension, abnormal cardiac looping and incomplete heart chamber formation. In higher magnification views, the morphants show that a small portion of GFP labeled cardiac cells (arrows in B′ to D′) did not migrate properly to be eventually packed in the heart tube. Dorsal view, head to left in A to C and A′ to C′. Lateral view, head to left in B to D and B′ to D′. Scale bar, 100 µm in A to C, and A′ to C′; 40 µm in D and D′. EXPRESSION / LABELING:
PHENOTYPE:
|
|
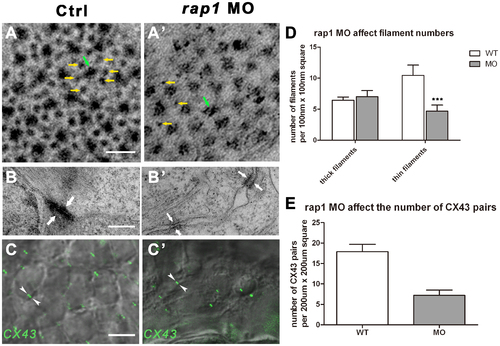
Ultra-structural changes of cell junctions and sarcomeres in Rap1 knock-down zebrafish heart. Transmission electronic scan revealed that the myofibril units, regularly organized into hexagonal lattices with thick (green arrow) and thin (yellow arrows) filaments (A) with adherens junctions (AJs) along the membrane of adjacent cardiac myocytes (B). In rap1MO heart, thick filaments (green arrow) number was not markablely changed but thin filaments (yellow arrows) number was significantly and statistically reduced (A′ and D), and less myofibrils attached to AJs (arrows in B and B′), with lager space between the membrane of adjacent cardiac myocytes (B′). Antibody against Connexin 43 stained a significantly and statistically less GJ signal (arrowheads in C and C′) in rap1MO cardiomyocytes at 3 dpf, compared to a normal heart (C, C′, and E). Scale bar, 50 nm in A and A′; 350 nm in B and B′; 50 µm in C and C′. Columns and error bars in D and E showed mean±S.D. n = 9 in filaments experiment (D) for each group, and n = 10 in C×43 antibody staining experiment (E) for each group. Unpaired two-tailed t-test was used to test the significance between two columns in each group in D. *** in statistic graph represent p<0.001 in the t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Rap1 knock-down zebrafish have prolonged P-R intervals, resembling the first-degree atrio-ventricular block. Zebrafish heart electrocardiography (ECG) was measured in wild type larvae (A), and rap1MO hearts at 3 dpf (B to D). PHENOTYPE:
|
|
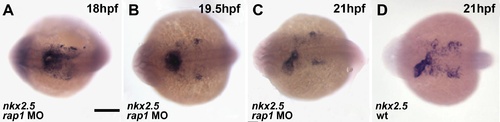
The expression of nkx2.5 in developing hearts. The cardiac primordial marker gene,nkx2.5, was expressed essentially normal between 18–21 hpf (A-C), compared to that of wild type heart (D) Dorsal view, anterior to the left. Scale bar, 200 µm. |
|
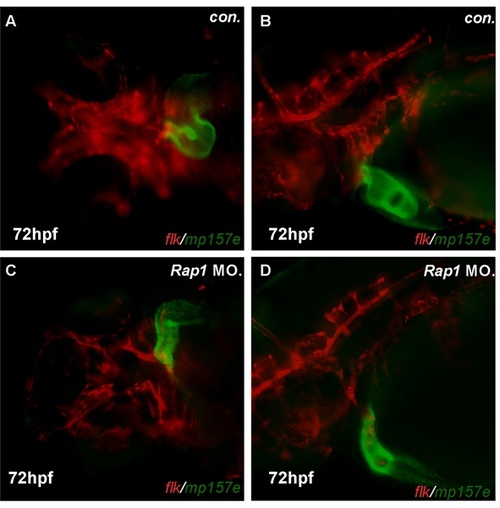
Morphant zebrafish has consistent defects in cardiac looping. Compared to control mp157e embryos (A, B), rap1MO injected mp157e embryos, crossed in flk1:mCherry background, showed faulty heart tube ‘S’ loop and thin cavity, in both ventral and lateral views (C,D). Shown are ventral view (A, C) and lateral view (B, D), with anterior to the left. PHENOTYPE:
|
|
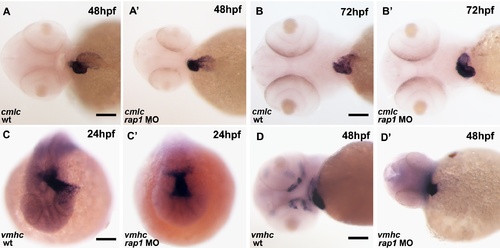
The cardiac looping defect was also evident by in situ hybridization. At 24 hpf, both control and rap1MO animals showed alike vmhc expression pattern in their hearts. Unlike the wild type hearts (A, C, D), the heart tube failed to extend left (C, C′) at 24 hpf or failed to form ‘S’ loop (A, A′, D and D′) in rap1MO animals at 48 hpf. At 72 hpf, ventricular defects, including pericardium edema and abnormal chamber differentiation, was observed in rap1MO (B, B′′). Ventral views were shown in A-A′, B-B′ and D-D′, anterior to the left;.dorsal view are shown in C-C′, anterior to the bottom. Scale bar, 150 μm in A, A′, D and D&prime& 120 μm in B and B&prime& 80 μm in C and C′. |

Unillustrated author statements PHENOTYPE:
|