- Title
-
Amyloid Beta precursor protein and prion protein have a conserved interaction affecting cell adhesion and CNS development
- Authors
- Kaiser, D.M., Acharya, M., Leighton, P.L., Wang, H., Daude, N., Wohlgemuth, S., Shi, B., and Allison, W.T.
- Source
- Full text @ PLoS One
|
Knockdown of Appa, Appb, or Prp1 results in impaired development and death of head region. A–L. Morpholino (MO) was delivered to disrupt translation of endogenous amyloid β precursor protein (APP) and prion protein (PrP) paralogs in zebrafish: appa, appb, or prp1 (top-bottom rows, respectively). Standard control MO at levels equivalent to our effective dose fail to induce any CNS cell death or disruptions in morphology of the fish (left column). Low doses of appa, appb, or prp1 MOs (0.5, 1.0, 0.5 ng respectively) were empirically determined to be sub-effective (Fig. S1,S2), leading to mild changes, but no death of CNS tissues (2nd column). Effective doses (1.0, 2.5, 1.0 ng, respectively) lead to severe alterations in CNS morphology (*) and death of CNS tissues (3rd column). Specificity of the MOs was demonstrated by rescuing the injection of an effective dose of appa, appb, or prp1 MO by co-injection of the cognate mRNA (200 pg, 200 pg or 100 pg, respectively; Right column). These data are quantified in Figs. 2F, S1 & S2. M. Western blots of zebrafish lysates reveal efficacy of our MO knockdown reagents (see also Fig. S3). The appa and appb splice blocking (SB) MOs used above (A–H) significantly decreases detection of protein species by the antibody 22C11 (top row). Bottom row is a β-actin loading control. An additional, independent MO that acts as a translation block of appa (appa TB) confirms this protein knockdown and produces similar phenotypes (Fig. S1). The prp1 MO reagents used here were previously shown to be effective in knocking down protein [27]. N. Quantification of western blots from three biological replicates (three independent injection trials on three separate days) demonstrate a significant decrease (*p<0.05, **p<0.01) of the APP immunoreactivity compared to β-actin with all three MO reagents at their effective doses. PHENOTYPE:
|
|
Appa and Appb can replace each other and thus are redundant in early zebrafish development. A–D Embryos were injected with sub-effective doses of appa and/or appb morpholino (MO). These doses produced no phenotype in the fish compared to control MO (A–C, see also Figure 1). D. When sub-effective doses of appa and appb MO were combined and injected, a strong phenotype emerged consisting of morphological malformations and death of tissues within the CNS (*). E. Quantifying this effect, the co-injection of sub-effective doses of both MOs produced a significant decrease in the number of normal fish (green bars) and a significant increase in number of fish displaying CNS cell death (mild in light orange bars; severe in dark orange bars). ** = P<0.01. F. Fish injected with appa MO can be rescued by co-injection with appa mRNA * = P<0.05. A similar result was attained for appb MO and its cognate mRNA (Fig. S2). G, H. Fish were injected with an effective dose of one MO along with cognate mRNA from the other paralog to see if rescue of the phenotype occurred. appb mRNA was able to effectively alleviate the phenotype caused by injection of the appa MO (G) and vice versa (H). ** = P<0.01. PHENOTYPE:
|
|
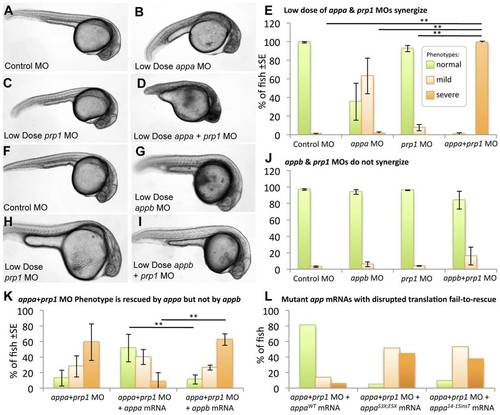
appa interacts with prp1, but appb does not. Panels A–E: Sub-effective doses of appa and prp1 gene knockdown synergize to produce an overt phenotype in the fish. Fish injected with a control morpholino (MO) (A), a sub-effective dose of appa (B) or prp1 (C) MO fail to display any signs of CNS cell death or disruptions in development, i.e. no severe phenotypes. D. When sub-effective doses of appa and prp1 are combined a severe phenotype emerges comprised of prominent morphological disruptions and an overt appearance of cell death within the CNS. E. The abundance of fish with normal morphology observed is significantly reduced, and the percentage of fish displaying cell death within the CNS is significantly increased when sub-effective doses of appa and prp1 MOs are combined. ** = P<0.01. Panels F–J present a similar experimental design to panels A–E, but represent appb knockdown instead of appa. When a sub-effective doses of appb and prp1 MOs are combined there is no significant increase in the number of fish showing developmental abnormalities or cell death within the CNS. K. Despite Appa and Appb being largely redundant during normal development (Fig. 2), they cannot replace each other when PrP1 abundance is reduced. appa mRNA is able to alleviate the phenotype caused by co-injection of sub-effective doses of appa and prp1 MOs. appa mRNA significantly reduced the percentage of fish displaying a severe phenotype. appb mRNA at an equivalent dose failed to reduce the percentage of fish displaying a phenotype. ** = P<0.01. L. app mRNAs with stop codon mutations are not able to rescue the app or appa+prp1 knockdown phenotypes. Data from the mutations S3X;E5X and 14_15 insT are shown (WT = wild type). Further analysis of these mRNAs and similar ones for appb was carried out in other knockdown backgrounds (Fig. S5). PHENOTYPE:
|
|
Apoptosis is synergistically increased when Appa and Prp1 levels are reduced. A–D. Zebrafish injected with a control morpholino (MO), low dose (sub-effective) prp1 MO, low dose (sub-effective) appa MO, or a combination of sub-effective appa and prp1 MOs (A–D, respectively) showed increased abundance of activated-caspase 3-positive cells (A′–D′, respectively). Higher doses of MOs used in this same assay showed individual MOs can also produce this effect (Fig. S7). E. Activated caspase 3-positive cells were slightly increased when low doses of prp1 or appa MOs were injected alone and synergistically increased when they were combined in one injection solution. N = 5. ** = P<0.01, * = P<0.05. PHENOTYPE:
|
|
Knockdown of APP and PrP synergize to reduce cell aggregation. Low doses of prp1 and appa knockdown reagents (morpholinos, MO) are used here to show that their effects on cell adhesion synergize; higher doses of MOs used in this same assay showed individual MOs can also produce this effect. A–D. Zebrafish embryos injected with fluorescent dyes along with control MO (A), low dose of prp1 MO (B), low dose of appa MO (C), or a combination of the two sub-effective MOs (D), were dissociated to single cells and subjected to an aggregation assay. Insets show clumped cells (or lack thereof) at higher magnification. E, F. Aliquots of dissociated cells taken prior to aggregation confirmed that dissociation was successful. G. The ability of these cells to form aggregates (10 or more cells in direct physical contact) rather than stay alone in solution was quantified. Cells with slightly reduced App or slightly reduced Prp had only marginal decreases in aggregation ability, whereas cells with both proteins reduced were significantly reduced in their aggregation ability. N = 3. * = P<0.05. PHENOTYPE:
|
|
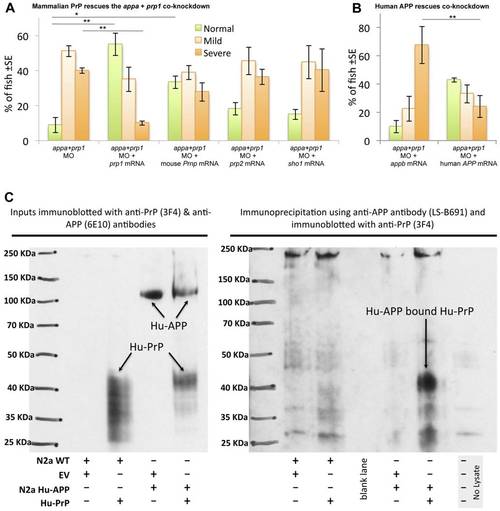
APP interactions with PrP are conserved from fish to mammals. A. Mouse Prnp can replace zebrafish prp1 in the context of its genetic interaction with appa. Co-injecting zebrafish prp1 mRNA, in concert with the Appa+Prp1 co-knockdown, rescues the observed phenotypes (first two sets of bars). prp1′s paralog, zebrafish prp2, does |
|
appa morpholino (MO) injection leads to a dose-dependent disruption in appa mRNA processing. A. Zebrafish embryos were injected with increasing doses of appa MO and scored for malformations and CNS cell death. B. Same experiments as panel A revealed doses that were toxic to the developing fish. C. appa splice block MO is efficacious, as it leads to disruption of appa mRNA. RNA was isolated from fish injected with appa MO, an equivalent dose of control MO, and subjected to RT-PCR. Fish injected with appa MO show a band at ~300 bp corresponding to mRNA with intron 2 retained. This band is absent in when fish are injected with the control MO, or when standard Taq is used in place of reverse-transcriptase. D. Sequencing of the aforementioned ~300 bp band confirms the retention of intron 2–3 in mature mRNA, and confirms our MO produces a truncated protein. Our sequence (top) was an exact match to zebrafish genomic clone NW_003336735 (bottom). Translation of the sequence, immediately 5′ of appa exon 3 (annotated in yellow at bottom right), predicts two termination codons (black, *). E–J. A second MO reagent against appa was used to test specificity of the phenotypes observed. Designed against a disparate portion of the gene, the 5′UTR and thus is a translation blocking (TB) MO. The efficacy of this MO is demonstrated in Fig. 1M and 1N. It produced mild and severe phenotypes (G and G′) indistinguishable from the splice blocking appa MO we primarily use in this work. The low dose of appa TB MO also showed a genetic interaction with the low dose of prp1 MO, equivalent to results in Fig. 3 (J, J′). MO dose is indicated on panels. K. Quantification of appa-TB MO demonstrates a dose-dependant effect by itself and an additive effect with prp1 MO during concerted delivery at sub-effective doses. Colour coding in the histogram is as per Fig. 2. |
|
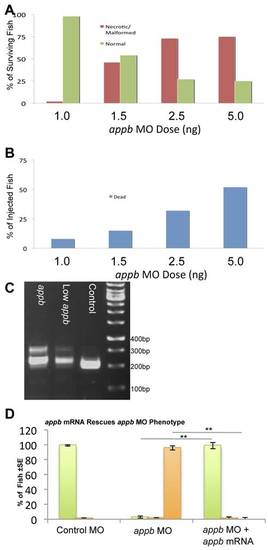
appb morpholino (MO) injection leads to a dose-dependent disruption in appb mRNA processing. A. Zebrafish embryos were injected with increasing doses of appb MO and screened based on presence of morphological malformations and CNS cell death. B. The same experiments as in panel A revealed doses that were toxic to the developing fish. C. appb splice block MO is efficacious, as it leads to disruption of appb mRNA. RNA was isolated from fish injected with 2.5 ng appb MO, 1.0 ng appb MO, or an equivalent dose of control MO, and subjected to RT-PCR. Fish injected with 2.5 ng of the appb MO show a band at ~300 bp corresponding to retention of intron 3–4 in mRNA. This band is reduced when the dose of the MO is reduced, and absent when fish are injected with the control MO. Sequencing of the band confirmed the retention of intron 3–4 in mature mRNA, and predicted STOP codons in the modified mRNA. D. Embryos injected with the appb MO alone or with 200 pg of cognate appb mRNA. The instance of fish displaying a severe phenotype was significantly reduced and the number of normal fish was significantly increased upon inclusion of appb mRNA. **p<0.01. Colour coding in the histogram D is as per Fig. 2 and Fig. S1. |
|
Efficacy of appa and appb MO’s assessed by western blot. MO knockdown reagents were assessed by Western blot to quantify protein abundance. Parts of this data appear in Fig. 1M. A. The size of the APP-immunoreactive bands in wild type mouse brain or from TgCRND8 mouse brains overexpressing human APP as detected with the antibody 22C11. B. Zebrafish App proteins are detected with 22C11 and the bands are indistinguishable from mammalian APP observed in panel A. Knockdown of zebrafish appa or appb gene products with various MO reagents (See Fig. 1, doses in nanograms are presented in brackets at the top of the figure) results in a significant reduction of APP immunoreactivity as normalized to β-actin levels and compared to control MO-injected fish. Smaller protein products that might be predicted to have a dominant effect following injection of splice blocking MOs are not detectable (predicted size of MO-altered proteins are 10 and 20 kDA for Appa and Appb, respectively). C. The 22C11 epitope (highlighted in blue) is conserved between human (top line) and zebrafish (Zf) proteins Appa (16/16 residues identical) and Appb (14/16 residues identical, 15/16 residues with conserved identity). Identity of the region is represented on the graph above the alignment with green showing perfect identity and amber showing mismatches. EXPRESSION / LABELING:
|
|
High doses of MOs affect apoptotic cell death, and Human APP rescues apoptotic cell death. High doses of prp1, appa or appb MO are used here to show their individual effects on apoptotic cell death. Some combinations of these MOs co-injected at low doses show that these MOs can synergize to produce this effect (Fig. 4). Apoptosis levels are increased when appa, appb, or prp1 mRNA is disrupted (A–D). Brightfield images of the area above the yolk sac extension of fish injected with effective doses of control, appb, appa and prp1 (A–D, respectively) MOs. Compared with control fish, prominent anti-activated caspase 3 staining is apparent in appb, appa, or prp1 MO injected fish (A′–D′, respectively). E. Number of caspase 3 positive cells were counted above the yolk sac extension in fish treated as per those in A–D. N = 5. Panels F–K show examples of human APP rescuing the concerted appa plus prp1 knockdown. appb is not able to rescue the phenotype in wildtype fish, nor in transgenic fish labelling the CNS with GFP (H & I, respectively, compare to F & G) as we noted in Fig. 3K and here serves as a negative control. A noticeable lack of GFP was apparent along portions of the CNS (* in panel I). Human APP is able to rescue these phenotypes (J, K). Panels L–N show the yolk-sac extension of fish in F, H & J. L′–N′ show examples of activated caspase labelling during rescue with human APP. The latter treatments were quantified in O. N = 5. * = P<0.05. ** = P<0.01. |