- Title
-
Rearrangements between differentiating hair cells coordinate planar polarity and the establishment of mirror symmetry in lateral-line neuromasts
- Authors
- Mirkovic, I., Pylawka, S., and Hudspeth, A.J.
- Source
- Full text @ Biol. Open
|
Rearrangements by pairs of newly formed hair cells. (A) In a schematic depiction of a neuromast, four mechanoreceptive hair cells are surrounded by nonsensory supporting, mantle, and periderm cells. The asymmetric position of the kinocilium demarcates each hair cell′s axis of planar polarity. (B) An apical view of a neuromast from a cldnB:lynGFP;ET4 doubly transgenic fish shows green fluorescence in the plasma membranes of all cells and in the cytoplasm of only the hair cells. This projection of several sections through the neuromast′s apex shows the actin-rich cuticular plates of hair cells (arrow). RFP-tagged centrin (red) labels centrosomes and indicates the orientation of each hair cell by marking the position of its kinociliary basal body (arrowheads). The neuromast′s plane of mirror symmetry is designated by the dotted line. In this and all subsequent figures the anterior direction is to the left and dorsal is upward. (C) In an image from a single plane in the same preparation, supporting cells and hair-cell precursors express only membrane-associated GFP and are morphologically indistinguishable. Cytoplasmic GFP demarcates the mature hair cells. (D) A time-lapse series focussed at or above the level of hair-cell nuclei documents the rearrangement of a pair of immature hair cells (arrowhead) in a cldnB:lynGFP;myo6b:mCherryCAAX larva. Rearrangements are specific to the daughter hair cells arising from a recent division and result in the mirror-symmetrical orientation of the pair. After completing their rearrangement, the maturing cells assume their final shapes (arrows). (E) The red channel of the foregoing time-lapse series visually isolates mature hair cells labeled with membrane-associated mCherry. Note the low level of labeling (arrow) in the newborn pair undergoing rearrangement. (F) The narrow apices of the immature pair of hair cells can first be observed in the third panel (arrows). As the apical membranes expand to encompass hair bundles, the orientations of the newly formed pair (arrowheads) can be determined from the positions of the openings in their cuticular plates. The scale bars represent 10μm in all panels. |
|
Normal cellular positioning and orientation in a neuromast. (A) The centrosomes of a dividing hair-cell precursor, which are marked red with centrin-RFP (arrowheads), rotate 90° to position the axis of division along the neuromast′s axis of polarity. After cytokinesis has been completed, rearrangement between the daughter cells commences. (B) A time-lapse series shows the rearrangement of a pair of hair cells following the division of a precursor orthogonally to a neuromast′s axis of polarity. An arrow in the first panel marks the position of one centrosome in a dividing hair-cell precursor; the other centrosome is out of the plane of focus. Apical movement of the centrosomes (arrowheads) coincides with the rearrangement, after which the hair-cell pair undergoes an additional 90° rotation to align with the neuromast′s axis of polarity. Note the rearrangement of a second pair of nascent hair cells at the dorsal pole of the neuromast. (C) A pair of supporting cells is formed through the symmetric division of a precursor positioned laterally to the central cluster of hair cells. The centrosomes in the precursor (arrows) move to position the axis of division obliquely relative to the neuromast′s axis of planar polarity. Although the centrosomes move apically (arrowheads), the supporting-cell pair does not undergo rearrangements. The scale bars represent 10μm in all panels. |
|
Hair-bundle misorientation in trilobite mutants. (A) A time-lapse series of a neuromast from a cldnB:lynGFP;tri larva demonstrates that nascent hair cells have an impaired capacity to complete rearrangements. Two precursor divisions, each marked by a pair of dots, occur simultaneously at the lateral edges of the neuromast. The divisions occur perpendicularly to the neuromast′s axis of polarity and parallel with its plane of mirror symmetry (dashed line). Although the daughter cells initiate rearrangements, they do not reverse positions. The scale bar represents 10μm. (B) Micrographs of the cellular apices taken at the end of the same recording document the final orientations of the four newly formed hair cells (arrowheads). The scale bar represents 5μm. (C) Phalloidin staining of a more mature neuromast shows the randomized orientation of mature hair cells in a trilobite mutant, which is schematized in the insert. The scale bar represents 5μm. Panels D–F provide quantification of hair-cell rearrangements under different experimental conditions and with various genetic backgrounds. All larvae, including those in control experiments, were exposed to 1% dimethyl sulfoxide, the solubilization vehicle for DAPT. (D) The percentage of position reversals between pairs of nascent hair cells differs strongly between 22 wild-type neuromasts (green) and 15 trilobite organs (red). The other experimental conditions are described in the text. (E) The average time devoted to rearrangements is significantly greater for 15 trilobite larvae than for 22 wild-type animals (p<0.01). The duration of rearrangements in animals treated with 100µM DAPT could not be accurately determined. (F) The orientation of divisions of hair-cell precursors relative to the neuromast′s polarity axis shows the profound effect of the trilobite mutation. |
|
Effects of DAPT treatment and loss of asymmetric Vangl2 localization. (A) Phalloidin staining (red) of a wild-type cldnB:lynGFP larva demonstrates the normal orientation of hair bundles in an anteroposteriorally polarized neuromast derived from the first primordium. This and the subsequent micrographs show apical views of neuromasts from cldnB:lynGFP larvae; the subjacent diagrams indicate the orientations of the hair cells. (B) The numerous hair cells in a neuromast of similar age treated with 100μM DAPT display a strong bias toward an anterior orientation. (C) After exposure to 100μM DAPT, the hair bundles in a neuromast derived from the second primordium show a striking dorsal bias. (D) A neuromast derived from the first primordium in a neurogenin1 larva displays an anterior bias after exposure to only 50μM DAPT. (E) Under control conditions, the production of hair cells (red) and supporting cells (blue) is relatively constant during an observation period beginning at 3 dpf. (F) In wild-type larvae a burst of hair-cell production begins 17–22 hr after the addition of 100μM DAPT; the differentiation of supporting cells is meanwhile suppressed. Note that imaging commenced 7 hr after exposure to 100μM DAPT. (G) In a neuromast of a myo6b:RFP-Vangl2;cldnB:lynGFP larva, Vangl2 (red) occurs in hair cells at the level of the apical actin belt surrounding the cuticular plate. An immature hair cell is labeled with an arrowhead. The top left inset shows the apices of the hair cells in the green channel. The lower inset in this and the subsequent illustrations schematizes the orientations of hair bundles. (H) Overexpression of Vangl2 eliminates the specific enrichment of the protein at the posterior apex and causes hair-cell misorientation. The mature hair cell with the strongest expression, which extends down the cell′s lateral borders (arrows), has a centrally placed kinocilium (arrowhead; dot in the inset). (I) Another neuromast with high levels of Vangl2 expression (red) displays a bias toward anterior orientations. (J) A neuromast from a cldnB:lynGFP;tri larva treated with 50μM DAPT and stained with phalloidin (red) contains hair cells with an anterior bias. The scale bars represent 10μm in all panels. |
|
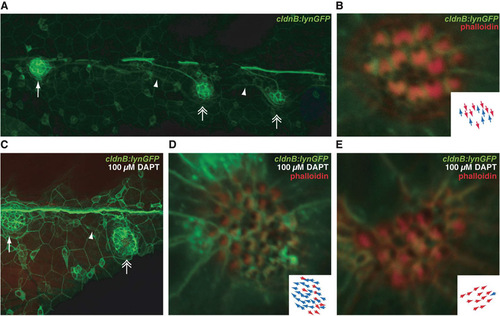
Effect of DAPT on neuromast orientation. (A) A low-magnification image shows one neuromast deposited by the first primordium (arrow) and two neuromasts produced by the second primordium (double arrows). Note the more ventral position of the latter neuromasts. Interneuromast cells of the first primordium displaced by the ventrally migrating neuromasts of the second primordium are marked with arrowheads. (B) A neuromast deposited by the second primordium displays a dorsoventral orientation. (C) A low-magnification image depicts neuromasts derived from the first (arrow) and second (double arrow) primordia in the posterior lateral line of a cldnB:lynGFP larva treated with 100 μM DAPT. The ventral migration of the second neuromast displaces a trail of interneuromast cells left by the first primordium (arrowhead). This indicates that positioning of the second primordium-derived neuromast relative to the anteroposterior and dorsoventral axes is not affected by DAPT treatment. (D) At a higher magnification, the neuromast derived from the second primordium displays hair cells oriented primarily along the anteroposterior axis and showing an anterior bias (inset). (E) In another preparation, the corresponding neuromast contains obliquely oriented hair cells and displays a posterodorsal bias (inset). |