- Title
-
RUNX3, EGR1 and SOX9B Form a Regulatory Cascade Required to Modulate BMP-Signaling during Cranial Cartilage Development in Zebrafish
- Authors
- Dalcq, J., Pasque, V., Ghaye, A., Larbuisson, A., Motte, P., Martial, J.A., and Muller, M.
- Source
- Full text @ PLoS One
|
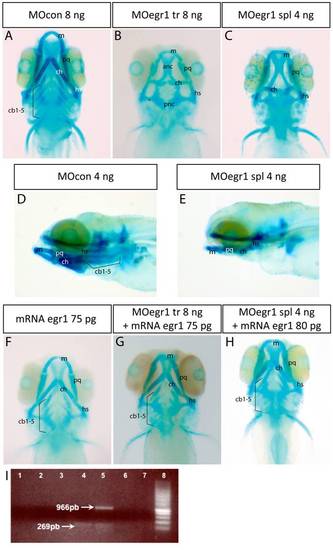
Knock-down of egr1 severely affects head cartilage formation at 4 dpf. (A-I) Head cartilages were stained with Alcian Blue in morpholino treated larvae at 4 dpf; ventral (A-C) and lateral (D,E) views are shown. (A,D) control 8 ng MOcon treated larvea. (B) 8 ng translation MOegr1 injected larveas display an absence of ceratobranchials and a reduction of size and mis-shaping of pharyngeal cartilage compared to controls (A); (C,E) 4ng splicing MOegr1 injected embryos display similar cartilage defects than 8 ng translation MOegr1 (B). (F) Ectopic expression of Egr1 does not significantly affect cartilage development. (G) Rescue of 8 ng MOegr1 tr treated larvae restores all cartilaginous elements of the viscerocranium. (H) A complete restoration of all cartilage elements is obtained by rescuing 4 ng splicing MOegr1 injected larvae. Meckel?s cartilage (m), palatoquadrate (pq), ceratohyal (ch) and hyosymplectic (hs), ceratobranchials 1 to 5 (cb1-5). (I) Agarose gel electrophoresis analysis of RT-PCR products from mRNA of injected embryos: 1) control mRNA; 2) mRNA of embryos injected with MOcon 4ng and without reverse transcriptase; 3) mRNA of embryos injected with MOegr1 spl 4 ng and without reverse transcriptase; 4) cDNA of embryos injected with MOcon 4 ng. Presence of a band at 269 bp, intron has been spliced properly; 5) cDNA of embryos injected with MOegr1 spl 4 ng. Presence of a band at 966 bp indicating that intron has not been properly spliced. However a residual band at 269 bp reveals that the mRNA has been partially spliced; 6) and 7) cDNA of MOcon 4 ng and MOegr1 spl 4 ng injected embryos that have not undergone the PCR step; 8) molecular weight marker. PHENOTYPE:
|
|
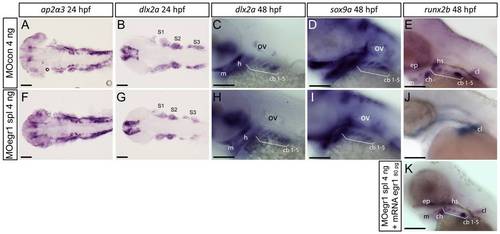
Only late chondrogenic and osteogenic marker genes display decreased expression in egr1 morphants between 24 and 48 hpf. In situ hybridization was performed at the indicated stages for various cartilage markers, lateral views, anterior to the left. Scale bars 100 μm. (A-E) 4 ng MOcon treated control embryos, (F,G,H,I,J) 4ng splicing MOegr1 injected embryos and (K) rescue. (A,F) At 24 hpf, ap2α3 expression in cranial neural crest cells (cNCC) is not altered in morphants. (B,C,G,H) cNCC marker dlx2a is normally expressed in egr1 morphants (G,H) compared to control embryos (B,C) at 24 and 48 hpf. (D,I) Expression of the essential chondrogenic gene sox9a is not changed at 48 hpf by egr1 knock-down. (E,J,K) At 48 hpf, runx2b transcripts are absent in pharyngeal cartilage precursor cells in 4 ng MOegr1 spl embryos. Expression of runx2b is maintained in the cleithrum (cl) and ethmoid plate (ep). (K) Rescue by injection of 80 pg mRNA egr1 restores all runx2b expression domains at 48 hpf. Otic vesicle (ov), mandible (m), ceratohyal (ch), hyosymplectic (hs), ceratobranchial pairs 1 to 5 (cb1-5), cleithrum (cl), ethmoid plate (ep), stream of cNCCs (S1-S3). |
|
Expression of egr1 in the pharyngeal region between 30 hpf to 5 dpf is restricted to endoderm and epithelium. Lateral (A-G,I) and ventral (H,J) views, anterior to the left. Scale bars 100 μm. Images of double in situ hybridizations were taken by confocal microscopy and pictures of individual Z-sections are shown. (A) egr1 transcripts are observed in the pharyngeal region starting at 30 hpf in endoderm. (B,C) At 48 hpf, double in situ hybridization for egr1 (green) and fli1 (red); egr1 transcripts are localized in pharyngeal endoderm and do not colocalize with fli1 mRNA in pharyngeal cartilage precursor cells. (D) egr1 is expressed in pharyngeal endoderm. (E-G) At 3 dpf, egr1 (green) does not colocalize with runx2b (red) (E) or sox9a (red) (F) in cartilage, while (G) egr1 (green) mRNAs colocalize with those for the pharyngeal endoderm marker sox9b (red). (H) At 4 dpf, egr1 (green) is never expressed in cells in pharyngeal cartilage precursor cells expressing fli1 (red). (I) Expression of egr1 at 4 dpf in pharyngeal endoderm. (J) At 5 dpf, egr1 is still expressed in pharyngeal endoderm (stars) and not in pharyngeal cartilage. Pharyngeal endoderm (pe), cranial neural crest cells (cNCC). |
|
Egr1 is required for expression of sox9b in pharyngeal endoderm. Endodermal gene expression by in situ hybridization (A,C,D,F,G,H,I) or in living transgenic embryos (B,E) in control embryos (A?C,H), egr1 morphants (D-F), rescued embryos (G) and sox9b mutants (I) at 48 hpf. Lateral views, anterior to the left. Scale bars 100 μm. (A,D) nkx2.3 expression is not altered in 4 ng MOegr1 spl injected embryos. (B,E) In living sox17:GFP transgenic embryos, the transgene is correctly expressed in egr1 morphants. (D,F,G) The endodermal marker sox9b is not expressed in the pharyngeal endoderm in 4 ng MOegr1 spl injected embryos, but its expression is rescued upon co-injection of 80 pg egr1 mRNA and spl 4 ng MOegr1. (H, I) In homozygous sox9b-/- embryos, egr1 transcripts are still observed in the pharyngeal endoderm like in the wild-type or heterozygous sox9b+/- embryos. Pharyngeal endoderm (pe), otic vesicle (ov). EXPRESSION / LABELING:
|
|
Runx3 is required for pharyngeal egr1 and sox9b expression at 48 hpf. Lateral views of in situ hybridizations (A,B,E-L) with indicated markers and ventral views of Alcian Blue stained embryos (C,D), anterior to the left. Scale bars 100 μm. (A,B) Endodermal runx3 expression in the pharyngeal region is not altered in 4 ng MOegr1 spl morphants. (C,D) runx3 knock-down using 2 ng MOrunx3 tr leads to total absence of viscerocranium and the anterior neurocranium (D) compared to control (C) embryos. (E,F) runx3 morphants do not express runx2b in pharyngeal cartilage precursor cells. (G,H) runx3 morphants do not express egr1 transcripts in pharyngeal endoderm. (I,J) The endodermal marker sox9b is absent in pharyngeal endoderm when runx3 expression is blocked. (K,L) runx3 knock-down does not affect expression of pharyngeal endodermal marker nkx2.3 at 48 hpf. Trigeminal ganglia (tg), pharyngeal endoderm (pe), cleithrum (cl), Meckel?s cartilage (m), palatoquadrate (pq), hyosymplectic (hs), ceratohyal (hs), ceratobranchials 1 to 5 (cb1-5), ethmoid plate (ep), otic vesivle (ov). |
|
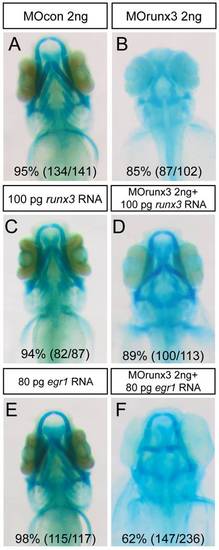
Runx3 depleted embryos can be rescued by runx3 and egr1 mRNA. (A-F) Head cartilages were stained with Alcian Blue in morpholino treated larvae at 4 dpf; ventral views are shown. (A) MOcon 2 ng injected larvae. (B) MOrunx3 2 ng injected larvae do not develop viscerocranium. (C) 100 pg of runx3 mRNA do not affect 4 dpf old larvae cartilage morphology. (D) Injection of 100 pg runx3 mRNA rescues 89% of MOrunx3 2 ng injected eggs. (E) 80 pg of egr1 mRNA. (F) Injection of 80 pg egr1 mRNA rescues 62% of MOrunx3 2 ng injected eggs. PHENOTYPE:
|
|
Expression of fsta is increased in runx3 and egr1 morphants and sox9b mutants. Lateral views of in situ hybridizations, anterior to the left. Scale bars 100 μm. (A-F) Compared to controls or wild-type embryos, expression of fsta is up-regulated in egr1 morphants (A,B), runx3 morphants (C,D), and homozygous sox9b mutants (E,F) at 48 hpf. pharyngeal endoderm (pe). (G,H) 4 dpf Alcian Blue stained larvae injected with MOcon 6 ng (K) and MOfsta 6ng (L). Knock-down of fsta causes a hyperplasia of the viscerocranium. Meckel?s cartilage (m), ceratohyal (ch), ceratobranchials 1 to 5 (cb1-5). |
|
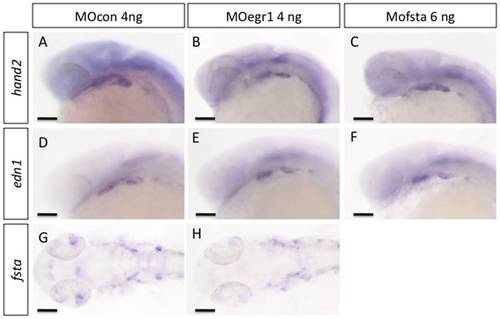
egr1 and fsta knock-down do not affect ventralisation of cranial neural crest cells. In situ hybridization was performed at 24 hpf, lateral views, anterior to the left. Scale bars 100 μm. (A,D,G) MOcon 4 ng, (B,E,H) MOegr1 4 ng and (C,F) MOfsta 6 ng. No modification in the expression of markers hand2, edn1 and fsta was observed in MOegr1 4 ng or MOfsta 6 ng injected embryos compared to control. EXPRESSION / LABELING:
|
|
BMP signaling is required between 27 and 37 hpf for runx2b expression and head cartilage development. (A-E) Cartilage was stained with Alcian Blue in 4 dpf larvae, ventral views are shown, anterior to the left. (A) Type 1 larvae (blue) display a wild-type morphology, all cartilage elements are present and well shapped. (B) Type 2 larvae (pink) lack ceratobranchials and have mis-shaped Meckel?s cartilage, palatoquadrate, ceratohyal and hyosymplectic. (C) Type 3 larvae (green) display a complete absence of viscerocranium and a reduction of the anterior neurocranium. (D,E) Graphs representing the proportions of the three types of larvae after the indicated treatments. (D) Treatment with the BMP inhibitor dorsomorphin (200 μM) most severely affects head cartilage between 29 and 45 hpf; (DMSO) dimethylsulfoxide. (E) Heat shock treatment of (hsp70l:dnBmpr-GFP)w30 transgenic embryos between 27 hpf and 37 hpf most severely affects pharyngeal cartilage development. (F-H) In situ hybridization for runx2b expression at 48 hpf, lateral views, anterior to the left, scale bars 100 μm. (F) Type 1 embryos (orange) have a normal runx2b expression pattern in all pharyngeal cartilage precursor cells, cleithrum and ethmoid plate. (G) Type 2 embryos (purple), compared to type 1 embryos, only express runx2b in cleithrum and weakly in the ethmoid plate. (H) Graph representing the proportions of the two types of larvae after the indicated treatments. Dorsomorphin treatment of wt embryos and heat shock treatment of (hsp70l:dnBmpr-GFP)w30 between 27 hpf and 37 hpf decreases runx2b expression in pharyngeal cartilage, but not in the cleithrum. (DMSO) dimethylsulfoxide, (Tg+) Transgene expressing embryo, (Tg-) Transgene non-expressing siblings, (HS) heat shock. Meckel?s cartilage (m), palatoquadrate (pq), ceratohyal (ch) and hyosymplectic (hs), ceratobranchials 1 to 5 (cb1-5). PHENOTYPE:
|
|
Bmp signaling is down-regulated in egr1 morphants. Pharyngeal cartilage precursor cells were visualized by immunohistochemistry using anti-GFP antibodies (green) in fli-GFP embryos. Activity of the BMP signaling pathway was assessed using antibodies against phospho-Smad1/5/8 (red) in 32 hpf embryos. Ventral view of pharyngeal arches, scale bar 40 μm. (A-F) Pharyngeal cartilage precursor cells were visualized by immunohistochemistry using anti-GFP antibodies (green) in fli1-GFP embryos. Activity of the BMP signaling pathway was assessed using antibodies against phospho-Smad1/5/8 (red) in 32 hpf embryos. Ventral view of pharyngeal arches, scale bar 40 μm. (A,B,C) 4 ng MOcon injected embryos, (D, E, F) 4 ng MOegr1 spl injected embryos. fli1-GFP embryos express the GFP transgene in cartilage precursors and endothelial cells in control (A) and in egr1 morphants (D). In contrast, phospho-Smad1/5/8 is is clearly down regulated in egr1 morphants (E) compared to control embryos (B). (C,F) Overlay images of the two anti-body signals clearly show that phospho-Smad1/5/8 is present in GFP-epressing cartilage precursor cells in control embryos (C), while no colocalization is observed in egr1 morphants (F). (a1) first arch, (a2) second arch, (a3) third arch, (a4) fourth arch, (bv) blood vessel. EXPRESSION / LABELING:
|
|
casanova mutants, lacking endoderm, do not express egr1 at 48 hpf. Single in situ hybridization for egr1 in casanova mutants. Lateral (A,B) and dorsal (C,D) views, anterior to the left. Scale bars 100 μm. (A,C) Wild-type or heterozygous cas+/- express egr1 in the pharyngeal endoderm (pe). (B,D) Homozygous cas-/- do not express egr1 in the pharyngeal region, but in the pectoral fins and in the two hearts of cas-/- embryos. Pectoral fin (pf), heart (h). |
|
casanova mutants, lacking endoderm, do not express sox9b or fsta at 48 hpf. Lateral views of in situ hybridizations (A-D) with indicated markers, anterior to the left. Scale bars 100 μm. Homozygous cas-/- do not express fsta (A,B) nor sox9b (C,D) compared to wild-type or heterozygous cas+/-. Pharyngeal endoderm (pe), otic vesicle (ov). |